The Presence of Yin-Yang Effects in the Migration Pattern of Staurosporine-Treated Single versus Collective Breast Carcinoma Cells
- PMID: 34769389
- PMCID: PMC8584475
- DOI: 10.3390/ijms222111961
The Presence of Yin-Yang Effects in the Migration Pattern of Staurosporine-Treated Single versus Collective Breast Carcinoma Cells
Abstract
Background: Staurosporine-dependent single and collective cell migration patterns of breast carcinoma cells MDA-MB-231, MCF-7, and SK-BR-3 were analysed to characterise the presence of drug-dependent migration promoting and inhibiting yin-yang effects.
Methods: Migration patterns of various breast cancer cells after staurosporine treatment were investigated using Western blot, cell toxicity assays, single and collective cell migration assays, and video time-lapse. Statistical analyses were performed with Kruskal-Wallis and Fligner-Killeen tests.
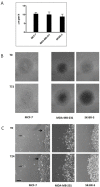
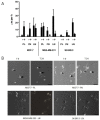
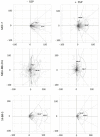
Results: Application of staurosporine induced the migration of single MCF-7 cells but inhibited collective cell migration. With the exception of low-density SK-BR-3 cells, staurosporine induced the generation of immobile flattened giant cells. Video time-lapse analysis revealed that within the borderline of cell collectives, staurosporine reduced the velocity of individual MDA-MB-231 and SK-BR-3, but not of MCF-7 cells. In individual MCF-7 cells, mainly the directionality of migration became disturbed, which led to an increased migration rate parallel to the borderline, and hereby to an inhibition of the migration of the cell collective as a total. Moreover, the application of staurosporine led to a transient activation of ERK1/2 in all cell lines.
Conclusion: Dependent on the context (single versus collective cells), a drug may induce opposite effects in the same cell line.
Keywords: breast carcinoma; cell migration; invasion; staurosporine; yin-yang effect.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

