Senolytic Therapy for Cerebral Ischemia-Reperfusion Injury
- PMID: 34769397
- PMCID: PMC8584561
- DOI: 10.3390/ijms222111967
Senolytic Therapy for Cerebral Ischemia-Reperfusion Injury
Abstract
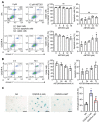
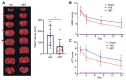
Ischemic stroke is one of the leading causes of death, and even timely treatment can result in severe disabilities. Reperfusion of the ischemic stroke region and restoration of the blood supply often lead to a series of cellular and biochemical consequences, including generation of reactive oxygen species (ROS), expression of inflammatory cytokines, inflammation, and cerebral cell damage, which is collectively called cerebral ischemia-reperfusion (IR) injury. Since ROS and inflammatory cytokines are involved in cerebral IR injury, injury could involve cellular senescence. Thus, we investigated whether senolytic therapy that eliminates senescent cells could be an effective treatment for cerebral IR injury. To determine whether IR induces neural cell senescence in vitro, astrocytes were subjected to oxygen-glucose deprivation/reoxygenation (OGD/R). OGD/R induced astrocyte senescence and senescent cells in OGD/R-injured astrocytes were effectively eliminated in vitro by ABT263, a senolytic agent. IR in rats with intraluminal middle cerebral artery occlusion induced cellular senescence in the ischemic region. The senescent cells in IR-injured rats were effectively eliminated by intravenous injections of ABT263. Importantly, ABT263 treatment significantly reduced the infarct volume and improved neurological function in behavioral tests. This study demonstrated, for the first time, that senolytic therapy has therapeutic potential for cerebral IR injury.
Keywords: ABT263; astrocyte; inflammation; ischemia-reperfusion injury; ischemic stroke; senescence; senolytic therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

