Single-Cell RNA Sequencing Reveals Heterogeneity and Functional Diversity of Lymphatic Endothelial Cells
- PMID: 34769408
- PMCID: PMC8584409
- DOI: 10.3390/ijms222111976
Single-Cell RNA Sequencing Reveals Heterogeneity and Functional Diversity of Lymphatic Endothelial Cells
Abstract
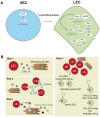
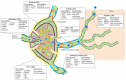
Lymphatic endothelial cells (LECs) line the lymphatic vasculature and play a central role in the immune response. LECs have abilities to regulate immune transport, to promote immune cell survival, and to cross present antigens to dendritic cells. Single-cell RNA sequencing (scRNA) technology has accelerated new discoveries in the field of lymphatic vascular biology. This review will summarize these new findings in regard to embryonic development, LEC heterogeneity with associated functional diversity, and interactions with other cells. Depending on the organ, location in the lymphatic vascular tree, and micro-environmental conditions, LECs feature unique properties and tasks. Furthermore, adjacent stromal cells need the support of LECs for fulfilling their tasks in the immune response, such as immune cell transport and antigen presentation. Although aberrant lymphatic vasculature has been observed in a number of chronic inflammatory diseases, the knowledge on LEC heterogeneity and functional diversity in these diseases is limited. Combining scRNA sequencing data with imaging and more in-depth functional experiments will advance our knowledge of LECs in health and disease. Building the case, the LEC could be put forward as a new therapeutic target in chronic inflammatory diseases, counterweighting the current immune-cell focused therapies.
Keywords: inflammation; lymph nodes; lymphatic endothelial cells; lymphatics; single cell RNA-sequencing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

