Identification of Spiro-Fused Pyrrolo[3,4- a]pyrrolizines and Tryptanthrines as Potential Antitumor Agents: Synthesis and In Vitro Evaluation
- PMID: 34769424
- PMCID: PMC8584944
- DOI: 10.3390/ijms222111997
Identification of Spiro-Fused Pyrrolo[3,4- a]pyrrolizines and Tryptanthrines as Potential Antitumor Agents: Synthesis and In Vitro Evaluation
Abstract
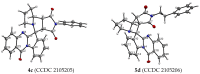
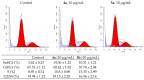
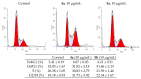
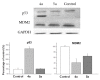
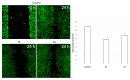
A series of heterocyclic compounds containing a spiro-fused pyrrolo[3,4-a]pyrrolizine and tryptanthrin framework have been synthesized and studied as potential antitumor agents. Cytotoxicity of products was screened against human erythroleukemia (K562) and human cervical carcinoma (HeLa) cell lines. Among the screened compounds. 4a, 4b and 5a were active against human erythroleukemia (K562) cell line, while 4a and 5a were active against cervical carcinoma (HeLa) cell line. In agreement with the DNA cytometry studies, the tested compounds have achieved significant cell-cycle perturbation with higher accumulation of cells in G2/M phase and induced apoptosis. Using confocal microscopy, we found that with 4a and 5a treatment of HeLa cells, actin filaments disappeared, and granular actin was distributed diffusely in the cytoplasm in 76-91% of cells. We discovered that HeLa cells after treatment with compounds 4a and 5a significantly reduced the number of cells with filopodium-like membrane protrusions (from 63 % in control cells to 29% after treatment) and a decrease in cell motility.
Keywords: 1,3-dipolar cycloaddition; antiproliferative activity; cell cycle; cell death; cell motility; morphological changes (cytoskeleton); one-pot synthesis; pyrrolo[3,4-a]pyrrolizine; tryptanthrin-derived azomethine ylide; tumor cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Lahlou M. The Success of Natural Products in Drug Discovery. Pharmacol. Pharm. 2013;4:17–31. doi: 10.4236/pp.2013.43A003. - DOI
-
- Srinivasulu V., Schilf P., Ibrahim S., Khanfar M.A., Sieburth S.M., Omar H., Sebastian A., AlQawasmeh R.A., O′Connor M.J., Al-Tel T.H. Multidirectional desymmetrization of pluripotent building block en route to diastereoselective synthesis of complex nature-inspired scaffolds. Nat. Commun. 2018;9:4989. doi: 10.1038/s41467-018-07521-2. - DOI - PMC - PubMed
-
- Fattorusso E., Taglialatela-Scafati O. Modern Alkaloids: Structure, Isolation, Synthesis and Biology. Wiley; Weinheim, Germany: 2008.

