Inhibitors of the Sec61 Complex and Novel High Throughput Screening Strategies to Target the Protein Translocation Pathway
- PMID: 34769437
- PMCID: PMC8585047
- DOI: 10.3390/ijms222112007
Inhibitors of the Sec61 Complex and Novel High Throughput Screening Strategies to Target the Protein Translocation Pathway
Abstract
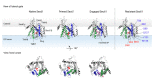
Proteins targeted to the secretory pathway start their intracellular journey by being transported across biological membranes such as the endoplasmic reticulum (ER). A central component in this protein translocation process across the ER is the Sec61 translocon complex, which is only intracellularly expressed and does not have any enzymatic activity. In addition, Sec61 translocon complexes are difficult to purify and to reconstitute. Screening for small molecule inhibitors impairing its function has thus been notoriously difficult. However, such translocation inhibitors may not only be valuable tools for cell biology, but may also represent novel anticancer drugs, given that cancer cells heavily depend on efficient protein translocation into the ER to support their fast growth. In this review, different inhibitors of protein translocation will be discussed, and their specific mode of action will be compared. In addition, recently published screening strategies for small molecule inhibitors targeting the whole SRP-Sec61 targeting/translocation pathway will be summarized. Of note, slightly modified assays may be used in the future to screen for substances affecting SecYEG, the bacterial ortholog of the Sec61 complex, in order to identify novel antibiotic drugs.
Keywords: Sec61 dependent translocation; co-translational translocation; endoplasmic reticulum; high throughput screening; inhibitor; signal recognition particle dependent protein targeting.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

