MATE-Type Proteins Are Responsible for Isoflavone Transportation and Accumulation in Soybean Seeds
- PMID: 34769445
- PMCID: PMC8585119
- DOI: 10.3390/ijms222112017
MATE-Type Proteins Are Responsible for Isoflavone Transportation and Accumulation in Soybean Seeds
Abstract
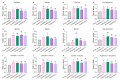
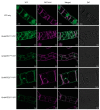
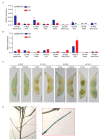
Soybeans are nutritionally important as human food and animal feed. Apart from the macronutrients such as proteins and oils, soybeans are also high in health-beneficial secondary metabolites and are uniquely enriched in isoflavones among food crops. Isoflavone biosynthesis has been relatively well characterized, but the mechanism of their transportation in soybean cells is largely unknown. Using the yeast model, we showed that GmMATE1 and GmMATE2 promoted the accumulation of isoflavones, mainly in the aglycone forms. Using the tobacco BrightYellow-2 (BY-2) cell model, GmMATE1 and GmMATE2 were found to be localized in the vacuolar membrane. Such subcellular localization supports the notion that GmMATE1 and GmMATE2 function by compartmentalizing isoflavones in the vacuole. Expression analyses showed that GmMATE1 was mainly expressed in the developing soybean pod. Soybean mutants defective in GmMATE1 had significantly reduced total seed isoflavone contents, whereas the overexpression of GmMATE1 in transgenic soybean promoted the accumulation of seed isoflavones. Our results showed that GmMATE1, and possibly also GmMATE2, are bona fide isoflavone transporters that promote the accumulation of isoflavones in soybean seeds.
Keywords: daidzein; genistein; glycitein; isoflavone; multidrug and toxic compound extrusion (MATE) transporter; seed; soybean.
Conflict of interest statement
The authors report no declarations of interest.
Figures
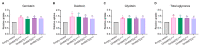
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

