Dental Pulp Stem Cell-Derived Secretome and Its Regenerative Potential
- PMID: 34769446
- PMCID: PMC8584775
- DOI: 10.3390/ijms222112018
Dental Pulp Stem Cell-Derived Secretome and Its Regenerative Potential
Abstract
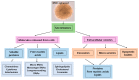
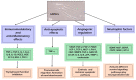
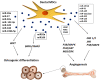
The therapeutic potential of the dental pulp stem (DSC) cell-derived secretome, consisting of various biomolecules, is undergoing intense research. Despite promising in vitro and in vivo studies, most DSC secretome-based therapies have not been implemented in human medicine because the paracrine effect of the bioactive factors secreted by human dental pulp stem cells (hDPSCs) and human exfoliated deciduous teeth (SHEDs) is not completely understood. In this review, we outline the current data on the hDPSC- and SHED-derived secretome as a potential candidate in the regeneration of bone, cartilage, and nerve tissue. Published reports demonstrate that the dental MSC-derived secretome/conditional medium may be effective in treating neurodegenerative diseases, neural injuries, cartilage defects, and repairing bone by regulating neuroprotective, anti-inflammatory, antiapoptotic, and angiogenic processes through secretome paracrine mechanisms. Dental MSC-secretomes, similarly to the bone marrow MSC-secretome activate molecular and cellular mechanisms, which determine the effectiveness of cell-free therapy. Many reports emphasize that dental MSC-derived secretomes have potential application in tissue-regenerating therapy due to their multidirectional paracrine effect observed in the therapy of many different injured tissues.
Keywords: dental stem cells; paracrine effect; regenerative medicine; secretome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

