PRDM12 in Health and Diseases
- PMID: 34769459
- PMCID: PMC8585061
- DOI: 10.3390/ijms222112030
PRDM12 in Health and Diseases
Abstract
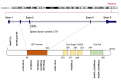
PRDM12 is a member of the PRDI-BF1 (positive regulatory domain I-binding factor 1) homologous domain (PRDM)-containing protein family, a subfamily of Kruppel-like zinc finger proteins, controlling key processes in the development of cancer. PRDM12 is expressed in a spatio-temporal manner in neuronal systems where it exerts multiple functions. PRDM12 is essential for the neurogenesis initiation and activation of a cascade of downstream pro-neuronal transcription factors in the nociceptive lineage. PRDM12 inactivation, indeed, results in a complete absence of the nociceptive lineage, which is essential for pain perception. Additionally, PRDM12 contributes to the early establishment of anorexigenic neuron identity and the maintenance of high expression levels of pro-opiomelanocortin, which impacts on the program bodyweight homeostasis. PRDMs are commonly involved in cancer, where they act as oncogenes/tumor suppressors in a "Yin and Yang" manner. PRDM12 is not usually expressed in adult normal tissues but its expression is re-activated in several cancer types. However, little information is currently available on PRDM12 expression in cancers and its mechanism of action has not been thoroughly described. In this review, we summarize the recent findings regarding PRDM12 by focusing on four main biological processes: neurogenesis, pain perception, oncogenesis and cell metabolism. Moreover, we wish to highlight the importance of future studies focusing on the PRDM12 signaling pathway(s) and its role in cancer onset and progression.
Keywords: PRD-BF1 and RIZ homology domain containing gene family; PRDM12; cancer; cell metabolism; neurogenesis; pain perception.
Conflict of interest statement
There are no competing financial interests in relation to the work described.
Figures
References
-
- Pinheiro I., Margueron R., Shukeir N., Eisold M., Fritzsch C., Richter F.M., Mittler G., Genoud C., Goyama S., Kurokawa M., et al. Prdm3 and Prdm16 are H3K9me1 Methyltransferases Required for Mammalian Heterochromatin Integrity. Cell. 2012;150:948–960. doi: 10.1016/j.cell.2012.06.048. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

