Dual Role of Thrombospondin-1 in Flow-Induced Remodeling
- PMID: 34769516
- PMCID: PMC8584526
- DOI: 10.3390/ijms222112086
Dual Role of Thrombospondin-1 in Flow-Induced Remodeling
Abstract
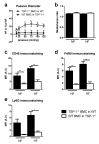
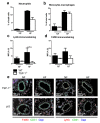
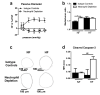
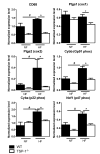
(1) Background: Chronic increases in blood flow, as in cardiovascular diseases, induce outward arterial remodeling. Thrombospondin-1 (TSP-1) is known to interact with matrix proteins and immune cell-surface receptors, but its contribution to flow-mediated remodeling in the microcirculation remains unknown. (2) Methods: Mesenteric arteries were ligated in vivo to generate high- (HF) and normal-flow (NF) arteries in wild-type (WT) and TSP-1-deleted mice (TSP-1-/-). After 7 days, arteries were isolated and studied ex vivo. (3) Results: Chronic increases in blood flow induced outward remodeling in WT mice (increasing diameter from 221 ± 10 to 280 ± 10 µm with 75 mmHg intraluminal pressure) without significant effect in TSP-1-/- (296 ± 18 to 303 ± 14 µm), neutropenic or adoptive bone marrow transfer mice. Four days after ligature, pro inflammatory gene expression levels (CD68, Cox2, Gp91phox, p47phox and p22phox) increased in WT HF arteries but not in TSP-1-/- mice. Perivascular neutrophil accumulation at day 4 was significantly lower in TSP-1-/- than in WT mice. (4) Conclusions: TSP-1 origin is important; indeed, circulating TSP-1 participates in vasodilation, whereas both circulating and tissue TSP-1 are involved in arterial wall thickness and diameter expansion.
Keywords: blood flow; immune cells; remodeling; resistance arteries; thrombospondin-1.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

