Pneumocystis jirovecii Pneumonia in Non-HIV Patients Recovering from COVID-19: A Single-Center Experience
- PMID: 34769913
- PMCID: PMC8582834
- DOI: 10.3390/ijerph182111399
Pneumocystis jirovecii Pneumonia in Non-HIV Patients Recovering from COVID-19: A Single-Center Experience
Abstract
Objective: to describe a single-center experience of Pneumocystis jirovecii pneumonia (PJP) in non-HIV patients recovering from COVID-19.
Methods: We report the cases of five non-HIV patients with COVID-19 who also developed PJP at a University Hospital.
Results: With the exception of one subject, who experienced an atypical and prolonged course of COVID-19, all the patients developed PJP after the clinical resolution of COVID-19 pneumonia. All but one patient had no pre-existing immunosuppressive conditions or other risk factors for PJP development at COVID-19 diagnosis. Nonetheless, following the course of COVID-19 infection, all the patients fulfilled at least one host factor for PJP; indeed, all the patients had received at least 2 weeks of high-dose steroids and three out of five had a CD4+ cell count <200/mm3.
Conclusions: The use of corticosteroids for COVID-19 respiratory impairment seems to be the most common risk factor for PJP, together with viral-induced and iatrogenic lymphopenia. The worsening in respiratory function and the characteristic radiological picture during or after COVID-19 pneumonia should raise the suspicion of PJP, even in immunocompetent patients. PJP primary chemoprophylaxis can be considered in selected high-risk COVID-19 patients, but further studies are needed.
Keywords: COVID-19; Pneumocystis jirovecii; corticosteroids; invasive fungal infections; lymphopenia.
Conflict of interest statement
The authors have no conflict of interest to disclose.
Figures
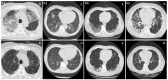
References
-
- Koehler P., Bassetti M., Chakrabarti A., Chen S.C.A., Colombo A.L., Hoenigl M., Klimko N., Lass-Flörl C., Oladele R.O., Vinh D.C., et al. Defining and managing COVID-19-associated pulmonary aspergillosis: The 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect. Dis. 2020;21:e149–e162. doi: 10.1016/S1473-3099(20)30847-1. - DOI - PMC - PubMed
-
- Donnelly J.P., Chen S.C., Kauffman C.A., Steinbach W.J., Baddley J.W., Verweij P., Clancy C.J., Wingard J.R., Lockhart S.R., Groll A.H., et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2019;71:1367–1376. doi: 10.1093/cid/ciz1008. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

