Diagnostic Accuracy of SARS-CoV-2 Antigen Tests for Community Transmission Screening: A Systematic Review and Meta-Analysis
- PMID: 34769968
- PMCID: PMC8583375
- DOI: 10.3390/ijerph182111451
Diagnostic Accuracy of SARS-CoV-2 Antigen Tests for Community Transmission Screening: A Systematic Review and Meta-Analysis
Abstract
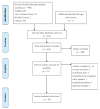
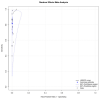
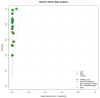
Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) caused the global pandemic of coronavirus disease 2019 (COVID-19). Rapid identification and isolation of infectious patients are critical methods to block COVID-19 transmission. Antigen tests can contribute to prompt identification of infectious individuals. This meta-analysis aims to evaluate the diagnostic accuracy of antigen tests for SARS-CoV-2. We conducted a literature search in PubMed, Embase, the Cochrane Library, and Biomed Central databases. Studies evaluating the diagnostic accuracy of antigen tests for SARS-CoV-2 in community participants were included. Only English-language articles were reviewed. We included eligible studies that provided available data to construct a 2 × 2 table on a per-patient basis. Overall sensitivity and specificity for antigen tests were generated using a bivariate random-effects model. Eighteen studies with 34,865 participants were retrieved. The meta-analysis for SARS-CoV-2 antigen tests generated a pooled sensitivity of 0.82 and a pooled specificity of 1.00. A subgroup analysis of ten studies that reported outcomes for 5629 symptomatic participants generated a pooled sensitivity of 0.87 and a pooled specificity of 1.00. Antigen tests might have higher sensitivity in detecting SARS-CoV-2 in symptomatic patients in the community and may be an effective tool to identify patients to be quarantined to prevent further SARS-CoV-2 transmission.
Keywords: COVID-19; SARS-CoV-2; antigen test; meta-analysis; sensitivity and specificity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Investigation of the diagnostic performance of the SARS-CoV-2 saliva antigen test: A meta-analysis.J Microbiol Immunol Infect. 2022 Dec;55(6 Pt 1):1084-1093. doi: 10.1016/j.jmii.2022.07.003. Epub 2022 Jul 16. J Microbiol Immunol Infect. 2022. PMID: 35922266 Free PMC article.
-
Diagnostic accuracy of SARS-CoV-2 antigen test in the pediatric population: A systematic review and meta-analysis.Pediatr Neonatol. 2023 May;64(3):247-255. doi: 10.1016/j.pedneo.2022.07.012. Epub 2022 Oct 25. Pediatr Neonatol. 2023. PMID: 36344413 Free PMC article.
-
Evaluation of the diagnostic accuracy of COVID-19 antigen tests: A systematic review and meta-analysis.J Chin Med Assoc. 2021 Nov 1;84(11):1028-1037. doi: 10.1097/JCMA.0000000000000626. J Chin Med Assoc. 2021. PMID: 34596082
-
Assessment of the detection accuracy of SARS-CoV-2 rapid antigen test in children and adolescents: An updated meta-analysis.J Chin Med Assoc. 2023 Nov 1;86(11):966-974. doi: 10.1097/JCMA.0000000000000987. Epub 2023 Sep 8. J Chin Med Assoc. 2023. PMID: 37683135
-
Thoracic imaging tests for the diagnosis of COVID-19.Cochrane Database Syst Rev. 2020 Sep 30;9:CD013639. doi: 10.1002/14651858.CD013639.pub2. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2020 Nov 26;11:CD013639. doi: 10.1002/14651858.CD013639.pub3. PMID: 32997361 Updated.
Cited by
-
Diagnostic Accuracy of Rapid Antigen Tests for COVID-19 Detection: A Systematic Review With Meta-analysis.Front Med (Lausanne). 2022 Apr 7;9:870738. doi: 10.3389/fmed.2022.870738. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35463027 Free PMC article.
-
Diagnostic Performance of Antigen Rapid Diagnostic Tests, Chest Computed Tomography, and Lung Point-of-Care-Ultrasonography for SARS-CoV-2 Compared with RT-PCR Testing: A Systematic Review and Network Meta-Analysis.Diagnostics (Basel). 2022 May 24;12(6):1302. doi: 10.3390/diagnostics12061302. Diagnostics (Basel). 2022. PMID: 35741112 Free PMC article. Review.
-
Comparative Evaluation of Rapid Isothermal Amplification and Antigen Assays for Screening Testing of SARS-CoV-2.Viruses. 2022 Feb 25;14(3):468. doi: 10.3390/v14030468. Viruses. 2022. PMID: 35336875 Free PMC article.
-
Investigation of the diagnostic performance of the SARS-CoV-2 saliva antigen test: A meta-analysis.J Microbiol Immunol Infect. 2022 Dec;55(6 Pt 1):1084-1093. doi: 10.1016/j.jmii.2022.07.003. Epub 2022 Jul 16. J Microbiol Immunol Infect. 2022. PMID: 35922266 Free PMC article.
-
Development of an Active Training Method for Belt Conveyor.Int J Environ Res Public Health. 2021 Dec 31;19(1):437. doi: 10.3390/ijerph19010437. Int J Environ Res Public Health. 2021. PMID: 35010694 Free PMC article.
References
-
- Lieberman J.A., Pepper G., Naccache S.N., Huang M.L., Jerome K.R., Greninger A.L. Comparison of Commercially Available and Laboratory-Developed Assays for In Vitro Detection of SARS-CoV-2 in Clinical Laboratories. J. Clin. Microbiol. 2020;58:e00821-20. doi: 10.1128/JCM.00821-20. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

