Charge Transfer Complexes of 1,3,6-Trinitro-9,10-phenanthrenequinone with Polycyclic Aromatic Compounds
- PMID: 34770800
- PMCID: PMC8588456
- DOI: 10.3390/molecules26216391
Charge Transfer Complexes of 1,3,6-Trinitro-9,10-phenanthrenequinone with Polycyclic Aromatic Compounds
Abstract
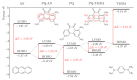
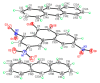
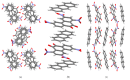
Understanding the interactions of organic donor and acceptor molecules in binary associates is crucial for design and control of their functions. Herein, we carried out a theoretical study on the properties of charge transfer complexes of 1,3,6-trinitro-9,10-phenanthrenequinone (PQ) with 23 aromatic π-electron donors. Density functional theory (DFT) was employed to obtain geometries, frontier orbital energy levels and amounts of charge transfer in the ground and first excited states. For the most effective donors, namely, dibenzotetrathiafulvalene, pentacene, tetrathiafulvalene, 5,10-dimethylphenazine, and tetramethyl-p-phenylenediamine, the amount of charge transfer in the ground state was shown to be 0.134-0.240 e-. Further, a novel charge transfer complex of PQ with anthracene was isolated in crystalline form and its molecular and crystal structure elucidated by single-crystal synchrotron X-ray diffraction.
Keywords: 1,3,6-trinitro-9,10-phenanthrenequinone; DFT; X-ray diffraction; anthracene; charge transfer complex.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
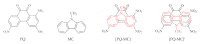
References
-
- Pavelyev V.G., Parashchuk O.D., Krompiec M., Orekhova T.V., Perepichka I.F., Van Loosdrecht P.H.M., Paraschuk D.Y., Pshenichnikov M.S. Charge transfer dynamics in donor-acceptor complexes between a conjugated polymer and fluorene acceptors. J. Phys. Chem. C. 2014;118:30291–30301. doi: 10.1021/jp510543c. - DOI
-
- Al Kobaisi M., Bhosale R.S., El-Khouly M.E., La D.D., Padghan S.D., Bhosale S.V., Jones L.A., Antolasic F., Fukuzumi S., Bhosale S.V. The sensitivity of donor – acceptor charge transfer to molecular geometry in DAN–NDI based supramolecular flower-like self-assemblies. Sci. Rep. 2017;7:16501. doi: 10.1038/s41598-017-15599-9. - DOI - PMC - PubMed
-
- Hu P., Du K., Wei F., Jiang H., Kloc C. Crystal Growth, HOMO–LUMO Engineering, and Charge Transfer Degree in Perylene-Fx TCNQ (x = 1, 2, 4) Organic Charge Transfer Binary Compounds. Cryst. Growth Des. 2016;16:3019–3027. doi: 10.1021/acs.cgd.5b01675. - DOI
-
- Singh M., Chopra D. Diversity in Mechanical Response in Donor–Acceptor Coupled Cocrystal Stoichiomorphs Based on Pyrene and 1,8-Dinitroanthraquinone Systems. Cryst. Growth Des. 2018;18:6670–6680. doi: 10.1021/acs.cgd.8b00918. - DOI
-
- Averkiev B., Isaac R., Jucov E.V., Khrustalev V.N., Kloc C., McNeil L.E., Timofeeva T.V. Evidence of Low-Temperature Phase Transition in Tetracene–Tetracyanoquinodimethane Complex. Cryst. Growth Des. 2018;18:4095–4102. doi: 10.1021/acs.cgd.8b00501. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

