A Novel Dual Organocatalyst for the Asymmetric Pinder Reaction and a Mechanistic Proposal Consistent with the Isoinversion Effect Thereof
- PMID: 34770807
- PMCID: PMC8588278
- DOI: 10.3390/molecules26216398
A Novel Dual Organocatalyst for the Asymmetric Pinder Reaction and a Mechanistic Proposal Consistent with the Isoinversion Effect Thereof
Abstract
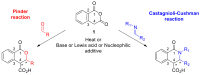
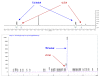
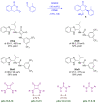
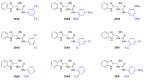
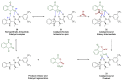
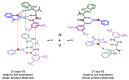
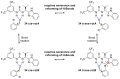
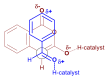
In general, the Pinder reaction concerns the reaction between an enolisable anhydride and an aldehyde proceeding initially through a Knoevenagel reaction followed by the ring closing process generating lactones with at least two chiral centers. These scaffolds are frequently present in natural products and synthetic bioactive molecules, hence it has attracted intense interest in organic synthesis and medicinal chemistry, particularly with respect to controlling the diastereo- and enantioselectivity. To the best of our knowledge, there has been only one attempt prior to this work towards the development of a catalytic enantioselective Pinder reaction. In our approach, we designed, synthesized, and tested dual chiral organocatalysts by combining BIMAH amines, (2-(α-(alkyl)methanamine)-1H-benzimidazoles, and a Lewis acid motif, such as squaramides, ureas and thioureas. The optimum catalyst was the derivative of isopropyl BIMAH bearing a bis(3,5-trifluoromethyl) thiourea, which afforded the Pinder products from various aromatic aldehydes with diastereomeric ratio >98:2 and enatioselectivity up to 92 ee%. Interestingly, the enantioselectivity of this catalyzed process is increased at higher concentrations and exhibits an isoinversion effect, namely an inverted "U" shaped dependency with respect to the temperature. Mechanistically, these features, point to a transition state involving an entropy-favored heterodimer interaction between a catalyst/anhydride and a catalyst/aldehyde complex when all other processes leading to this are much faster in comparison above the isoinversion temperature.
Keywords: asymmetric organocatalysis; castagnolli–cushman reaction; chiral thiourea-BiMAH catalysts; isoinversion temperature; pinder reaction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




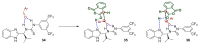
References
-
- Perkin W.H.J.J. XI.—On the formation of coumarin and of cinnamic and of other analogous acids from the aromatic aldehydes. Chem. Soc. 1877;31:388–427. doi: 10.1039/JS8773100388. - DOI
-
- Fittig R., Jayne H.W. Ueber das Phenyl-Butyrolacton und die Phenyl-Paraconsäure. Justus Liebigs Ann. Chem. 1883;216:97.
-
- Jones J.B., Pinder A.R.J. Some synthetical investigations in isocoumarin chemistry. Chem. Soc. 1958;531:2612–2618. doi: 10.1039/jr9580002612. - DOI
-
- Yu N., Poulain R., Tartar A., Gesquiere J.-C. Cycloaddition of homophthalic anhydrides with aldehydes and ketones: A route to 3,4-dihydroisocoumarin-4-carboxylic acid derivatives. Tetrahedron. 1999;55:13735–13740. doi: 10.1016/S0040-4020(99)00840-6. - DOI
-
- Bogdanov M.G., Palamareva M.D. cis/trans-Isochromanones. DMAP induced cycloaddition of homophthalic anhydride and aldehydes. Tetrahedron. 2004;60:2525. doi: 10.1016/j.tet.2004.01.040. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

