Phytochemistry and Pharmacological Activities of the Diterpenoids from the Genus Daphne
- PMID: 34771007
- PMCID: PMC8588408
- DOI: 10.3390/molecules26216598
Phytochemistry and Pharmacological Activities of the Diterpenoids from the Genus Daphne
Abstract
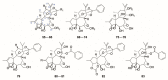
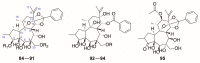
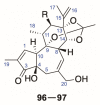
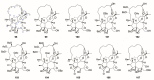
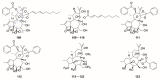
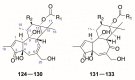
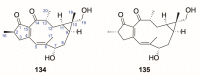
There are abundant natural diterpenoids in the plants of the genus Daphne from the Thymelaeaceae family, featuring a 5/7/6-tricyclic ring system and usually with an orthoester group. So far, a total of 135 diterpenoids has been isolated from the species of the genus Daphne, which could be further classified into three main types according to the substitution pattern of ring A and oxygen-containing functions at ring B. A variety of studies have demonstrated that these compounds exert a wide range of bioactivities both in vitro and in vivo including anticancer, anti-inflammatory, anti-HIV, antifertility, neurotrophic, and cholesterol-lowering effects, which is reviewed herein. Meanwhile, the fascinating structure-activity relationship is also concluded in this review in the hope of providing an easy access to available information for the synthesis and optimization of efficient drugs.
Keywords: Daphne; bioactivities; diterpenoid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

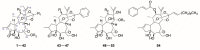

References
-
- Editorial Committee of Chinese Academy of Sciences . Flora Reipublicae Popularis Sinicae. Volume 52. Science Press; Peking, China: 1999. The genus Daphne Linn; p. 331.
Publication types
MeSH terms
Substances
Grants and funding
- 81703672/National Natural Science Foundation of the People's Republic of China
- PWRq2020-66/Excellent Youth Medical Talents Training Program of Pudong Health Bureau of Shanghai
- PDZY-2018-0604/Clinical Chinese Medicine Plateau Discipline Construction Project of Shanghai Pudong New Area Health Committee
- PWXx2020-04/New Interdisciplinary Subjects of Pudong New Area Health Committee
- PDZY-2018-0801/Pudong New Area Special Research and Innovation of Chinese Medicine
LinkOut - more resources
Full Text Sources
Medical

