Mesoporous Poly(melamine- co-formaldehyde) Particles for Efficient and Selective Phosphate and Sulfate Removal
- PMID: 34771024
- PMCID: PMC8588240
- DOI: 10.3390/molecules26216615
Mesoporous Poly(melamine- co-formaldehyde) Particles for Efficient and Selective Phosphate and Sulfate Removal
Abstract
Due to the existence-threatening risk to aquatic life and entire ecosystems, the removal of oxyanions such as sulfate and phosphate from anthropogenic wastewaters, such as municipal effluents and acid mine drainage, is inevitable. Furthermore, phosphorus is an indispensable resource for worldwide plant fertilization, which cannot be replaced by any other substance. This raises phosphate to one of the most important mineral resources worldwide. Thus, efficient recovery of phosphate is essential for ecosystems and the economy. To face the harsh acidic conditions, such as for acid mine drainage, an adsorber material with a high chemical resistivity is beneficial. Poly(melamine-co-formaldehyde) (PMF) sustains these conditions whilst its very high amount of nitrogen functionalities (up to 53.7 wt.%) act as efficient adsorption sides. To increase adsorption capacities, PMF was synthesized in the form of mesoporous particles using a hard-templating approach yielding specific surface areas up to 409 m2/g. Different amounts of silica nanospheres were utilized as template and evaluated for the adsorption of sulfate and phosphate ions. The adsorption isotherms were validated by the Langmuir model. Due to their properties, the PMF particles possessed outperforming maximum adsorption capacities of 341 and 251 mg/g for phosphate and sulfate, respectively. Furthermore, selective adsorption of sulfate from mixed solutions of phosphate and sulfate was found for silica/PMF hybrid particles.
Keywords: hard templating; melamine–formaldehyde resin; oxyanion removal; porous resin particles; selectivity; silica; sorption; water treatment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
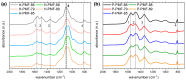
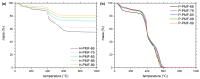

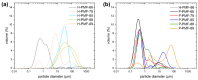
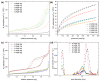
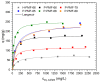

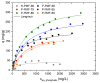

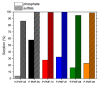

References
-
- Fatima T., Muntean A. Sulfate attack in sewer pipes: Derivation of a concrete corrosion model via two-scale convergence. Nonlinear Anal. Real World Appl. 2014;15:326–344. doi: 10.1016/j.nonrwa.2012.01.019. - DOI
-
- Katayev E.A., Ustynyuk Y.A., Sessler J.L. Receptors for tetrahedral oxyanions. Coord. Chem. Rev. 2006;250:3004–3037. doi: 10.1016/j.ccr.2006.04.013. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

