Globally Approved EGFR Inhibitors: Insights into Their Syntheses, Target Kinases, Biological Activities, Receptor Interactions, and Metabolism
- PMID: 34771085
- PMCID: PMC8587155
- DOI: 10.3390/molecules26216677
Globally Approved EGFR Inhibitors: Insights into Their Syntheses, Target Kinases, Biological Activities, Receptor Interactions, and Metabolism
Abstract
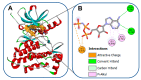
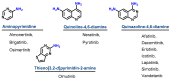
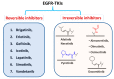
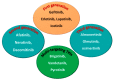
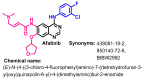
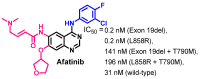
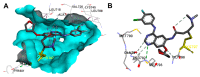
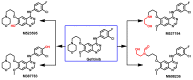
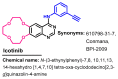
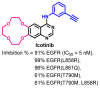
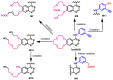
Targeting the EGFR with small-molecule inhibitors is a confirmed valid strategy in cancer therapy. Since the FDA approval of the first EGFR-TKI, erlotinib, great efforts have been devoted to the discovery of new potent inhibitors. Until now, fourteen EGFR small-molecule inhibitors have been globally approved for the treatment of different types of cancers. Although these drugs showed high efficacy in cancer therapy, EGFR mutations have emerged as a big challenge for these drugs. In this review, we focus on the EGFR small-molecule inhibitors that have been approved for clinical uses in cancer therapy. These drugs are classified based on their chemical structures, target kinases, and pharmacological uses. The synthetic routes of these drugs are also discussed. The crystal structures of these drugs with their target kinases are also summarized and their bonding modes and interactions are visualized. Based on their binding interactions with the EGFR, these drugs are also classified into reversible and irreversible inhibitors. The cytotoxicity of these drugs against different types of cancer cell lines is also summarized. In addition, the proposed metabolic pathways and metabolites of the fourteen drugs are discussed, with a primary focus on the active and reactive metabolites. Taken together, this review highlights the syntheses, target kinases, crystal structures, binding interactions, cytotoxicity, and metabolism of the fourteen globally approved EGFR inhibitors. These data should greatly help in the design of new EGFR inhibitors.
Keywords: EGFR; anticancer; kinase inhibitor; metabolism; synthesis.
Conflict of interest statement
Authors declare that there is no conflict of interest and have approved the manuscript.
Figures



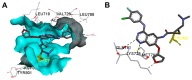





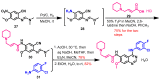
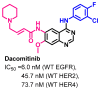

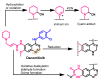
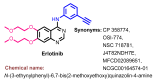
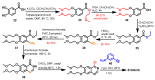
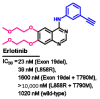
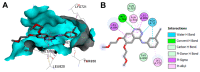
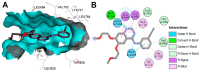
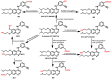
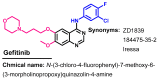
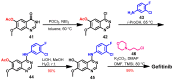


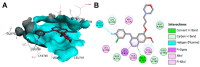


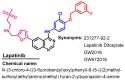
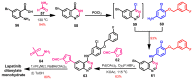
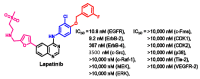




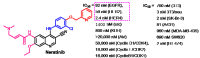
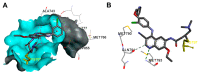
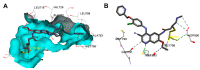
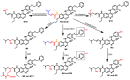
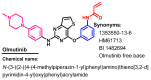
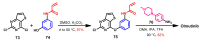
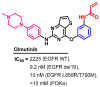

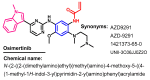

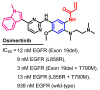



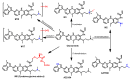
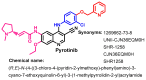

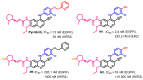



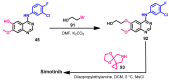
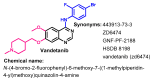
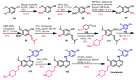
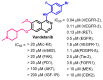
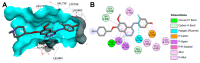
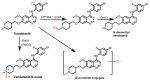
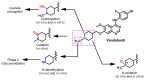
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

