Development of Triazoles and Triazolium Salts Based on AZT and Their Anti-Viral Activity against HIV-1
- PMID: 34771129
- PMCID: PMC8588071
- DOI: 10.3390/molecules26216720
Development of Triazoles and Triazolium Salts Based on AZT and Their Anti-Viral Activity against HIV-1
Abstract
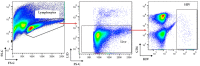
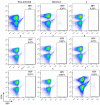
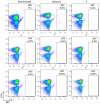
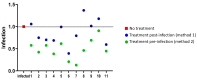
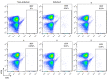
We report herein a set of 3'-azido-3'-deoxythymidine (AZT) derivatives based on triazoles and triazolium salts for HIV-1 infection. The compounds were synthesized via click chemistry with Cu(I) and Ru(II) catalysts. Triazolium salts were synthesized by reaction with methyl iodide or methyl triflate in good yields. The antiviral activity of the compounds was tested using two methodologies: In method one the activity was measured on infected cells; in method two a pre-exposure prophylaxis experimental model was employed. For method one the activity of the compounds was moderate, and in general the triazolium salts showed a decreased activity in relation to their triazole precursors. With method two the antiviral activity was higher. All compounds were able to decrease the infection, with two compounds able to clear almost all the infection, while a lower antiviral activity was noted for the triazolium salts. These results suggest that these drugs could play an important role in the development of pre-exposure prophylaxis therapies.
Keywords: AZT; HIV-1; anti-viral; click chemistry; triazoles; triazolium salts.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


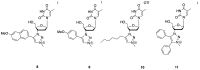
Similar articles
-
Antiretroviral activity and cytotoxicity of novel zidovudine (AZT) derivatives and the relation to their chemical structure.Int J Antimicrob Agents. 2002 Oct;20(4):282-8. doi: 10.1016/s0924-8579(02)00191-7. Int J Antimicrob Agents. 2002. PMID: 12385685
-
Clicking 3'-azidothymidine into novel potent inhibitors of human immunodeficiency virus.J Med Chem. 2013 Nov 14;56(21):8765-80. doi: 10.1021/jm401232v. Epub 2013 Oct 28. J Med Chem. 2013. PMID: 24102161 Free PMC article.
-
Synthesis and antiviral activity of amino acid carbamate derivatives of AZT.Nucleosides Nucleotides Nucleic Acids. 2000 Jan-Feb;19(1-2):87-100. doi: 10.1080/15257770008032998. Nucleosides Nucleotides Nucleic Acids. 2000. PMID: 10772704
-
Novel approaches for designing 5'-O-ester prodrugs of 3'-azido-2', 3'-dideoxythymidine (AZT).Curr Med Chem. 2000 Oct;7(10):995-1039. doi: 10.2174/0929867003374372. Curr Med Chem. 2000. PMID: 10911016 Review.
-
Research Advances on the Bioactivity of 1,2,3-Triazolium Salts.Int J Mol Sci. 2023 Jun 27;24(13):10694. doi: 10.3390/ijms241310694. Int J Mol Sci. 2023. PMID: 37445872 Free PMC article. Review.
Cited by
-
Novel pyrrole based triazole moiety as therapeutic hybrid: synthesis, characterization and anti-Alzheimer potential with molecular mechanism of protein ligand profile.BMC Chem. 2024 Nov 9;18(1):223. doi: 10.1186/s13065-024-01340-x. BMC Chem. 2024. PMID: 39522005 Free PMC article.
-
A Literature Review Focusing on the Antiviral Activity of [1,2,4] and [1,2,3]-triazoles.Mini Rev Med Chem. 2024;24(17):1602-1629. doi: 10.2174/0113895575277122231108095511. Mini Rev Med Chem. 2024. PMID: 38008942 Review.
-
Click Chemistry: an overview and recent updates in the medicinal attributes of click-derived heterocycles.Mol Divers. 2025 Feb 7. doi: 10.1007/s11030-025-11110-z. Online ahead of print. Mol Divers. 2025. PMID: 39918713 Review.
References
MeSH terms
Substances
Grants and funding
- UIDB/04612/2020/Fundação para a Ciência e Tecnologia
- UIDP/04612/2020/Fundação para a Ciência e Tecnologia
- IF/00109/2014/CP1244/CT0007/Fundação para a Ciência e Tecnologia
- IF/01722/2013/Fundação para a Ciência e Tecnologia
- CEECIND/01049/2020/Fundação para a Ciência e Tecnologia
- PD/BD/128343/2017/Fundação para a Ciência e Tecnologia
- SFRH/BD/135296/2017/Fundação para a Ciência e Tecnologia
- SFRH/BPD/124739/2016/Fundação para a Ciência e Tecnologia
- PD/BD/135483/2018/Fundação para a Ciência e Tecnologia
- SFRH/BD/1444412019/Fundação para a Ciência e Tecnologia
- PGG 009/2017/Gilead Génese
- NOVASAUDE 2017/Universidade Nova de Lisboa
- CEECINST/001202/2018/Fundação para a Ciência e Tecnologia
- n/a/European Society of Clinical Microbiology and Infectious Diseases
LinkOut - more resources
Full Text Sources
Medical

