Development of Bio-Composites with Enhanced Antioxidant Activity Based on Poly(lactic acid) with Thymol, Carvacrol, Limonene, or Cinnamaldehyde for Active Food Packaging
- PMID: 34771206
- PMCID: PMC8588526
- DOI: 10.3390/polym13213652
Development of Bio-Composites with Enhanced Antioxidant Activity Based on Poly(lactic acid) with Thymol, Carvacrol, Limonene, or Cinnamaldehyde for Active Food Packaging
Abstract
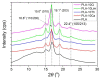
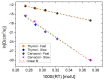
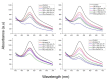
The new trend in food packaging films is to use biodegradable or bio-based polymers, such as poly(lactic acid), PLA with additives such as thymol, carvacrol, limonene or cinnamaldehyde coming from natural resources (i.e., thyme, oregano, citrus fruits and cinnamon) in order to extent foodstuff shelf-life and improve consumers' safety. Single, triple and quadruple blends of these active compounds in PLA were prepared and studied using the solvent-casting technique. The successful incorporation of the active ingredients into the polymer matrix was verified by FTIR spectroscopy. XRD and DSC data revealed that the crystallinity of PLA was not significantly affected. However, the Tg of the polymer decreased, verifying the plasticization effect of all additives. Multicomponent mixtures resulted in more intense plasticization. Cinnamaldehyde was found to play a catalytic role in the thermal degradation of PLA shifting curves to slightly lower temperatures. Release of thymol or carvacrol from the composites takes place at low rates at temperatures below 100 °C. A combined diffusion-model was found to simulate the experimental release profiles very well. Higher antioxidant activity was noticed when carvacrol was added, followed by thymol and then cinnamaldehyde and limonene. From the triple-component composites, higher antioxidant activity measured in the materials with thymol, carvacrol and cinnamaldehyde.
Keywords: antioxidant properties; biobased polymers; carvacrol; cinnamaldehyde; food packaging; limonene; poly (lactic acid) PLA; thymol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
















Similar articles
-
Effect of PLA Active Packaging Containing Monoterpene-Cyclodextrin Complexes on Berries Preservation.Polymers (Basel). 2021 Apr 26;13(9):1399. doi: 10.3390/polym13091399. Polymers (Basel). 2021. PMID: 33925969 Free PMC article.
-
Development of novel nano-biocomposite antioxidant films based on poly (lactic acid) and thymol for active packaging.Food Chem. 2014 Nov 1;162:149-55. doi: 10.1016/j.foodchem.2014.04.026. Epub 2014 Apr 13. Food Chem. 2014. PMID: 24874370
-
Development and Characterization of a Biodegradable PLA Food Packaging Hold Monoterpene-Cyclodextrin Complexes against Alternaria alternata.Polymers (Basel). 2019 Oct 21;11(10):1720. doi: 10.3390/polym11101720. Polymers (Basel). 2019. PMID: 31640138 Free PMC article.
-
Bio-based active food packaging materials: Sustainable alternative to conventional petrochemical-based packaging materials.Food Res Int. 2020 Nov;137:109625. doi: 10.1016/j.foodres.2020.109625. Epub 2020 Aug 14. Food Res Int. 2020. PMID: 33233213 Review.
-
A review of poly(lactic acid)-based materials for antimicrobial packaging.J Food Sci. 2014 Aug;79(8):R1477-90. doi: 10.1111/1750-3841.12534. Epub 2014 Jul 17. J Food Sci. 2014. PMID: 25039867 Review.
Cited by
-
Improving the antibacterial properties of polyethylene food packaging films with Ajwain essential oil adsorbed on chitosan particles.Sci Rep. 2024 Nov 20;14(1):28802. doi: 10.1038/s41598-024-80349-7. Sci Rep. 2024. PMID: 39567677 Free PMC article.
-
Biological Activity of Monoterpene-Based Scaffolds: A Natural Toolbox for Drug Discovery.Molecules. 2025 Mar 27;30(7):1480. doi: 10.3390/molecules30071480. Molecules. 2025. PMID: 40286078 Free PMC article. Review.
-
Innovative Materials Based on Epoxy Resin for Use as Seat Elements in Bulk Transport.Materials (Basel). 2024 Apr 16;17(8):1829. doi: 10.3390/ma17081829. Materials (Basel). 2024. PMID: 38673186 Free PMC article.
-
Release and Disintegration Properties of Poly(lactic Acid) Films with Allyl Isothiocyanate-β-Cyclodextrin Inclusion Complexes for Active Food Packaging.Molecules. 2024 Dec 12;29(24):5859. doi: 10.3390/molecules29245859. Molecules. 2024. PMID: 39769948 Free PMC article.
-
Origanum vulgare ssp. hirtum (Lamiaceae) Essential Oil Prevents Behavioral and Oxidative Stress Changes in the Scopolamine Zebrafish Model.Molecules. 2021 Nov 23;26(23):7085. doi: 10.3390/molecules26237085. Molecules. 2021. PMID: 34885665 Free PMC article.
References
-
- Vermeiren L., Devlieghere F., van Beest M., de Kruijf N., Debevere J. Developments in the active packaging of foods. Trends Food Sci. Technol. 1999;10:77–86. doi: 10.1016/S0924-2244(99)00032-1. - DOI
-
- Appendini P., Hotchkiss J.H. Review of antimicrobial food packaging. Innov. Food Sci. Emerg. Technol. 2002;3:113–126. doi: 10.1016/S1466-8564(02)00012-7. - DOI
-
- Suppakul P., Miltz J., Sonneveld K., Bigger S.W. Active packaging Technologies with an Emphasis on Antimicrobial Packaging and its Applications. J. Food Sci. 2003;68:408–420. doi: 10.1111/j.1365-2621.2003.tb05687.x. - DOI
-
- Persico P., Ambrogi V., Carfagna C., Cerruti P., Ferrocino I., Mauriello G. Nanocomposite polymer films containing carvacrol for antimicrobial active packaging. Polym. Eng. Sci. 2009;49:1447–1455. doi: 10.1002/pen.21191. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

