Advanced Carbon Materials Derived from Polybenzoxazines: A Review
- PMID: 34771331
- PMCID: PMC8587001
- DOI: 10.3390/polym13213775
Advanced Carbon Materials Derived from Polybenzoxazines: A Review
Abstract
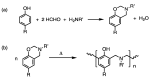
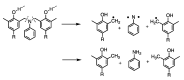
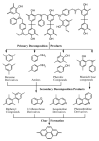
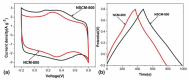
This comprehensive review article summarizes the key properties and applications of advanced carbonaceous materials obtained from polybenzoxazines. Identification of several thermal degradation products that arose during carbonization allowed for several different mechanisms (both competitive ones and independent ones) of carbonization, while also confirming the thermal stability of benzoxazines. Electrochemical properties of polybenzoxazine-derived carbon materials were also examined, noting particularly high pseudocapacitance and charge stability that would make benzoxazines suitable as electrodes. Carbon materials from benzoxazines are also highly versatile and can be synthesized and prepared in a number of ways including as films, foams, nanofibers, nanospheres, and aerogels/xerogels, some of which provide unique properties. One example of the special properties is that materials can be porous not only as aerogels and xerogels, but as nanofibers with highly tailorable porosity, controlled through various preparation techniques including, but not limited to, the use of surfactants and silica nanoparticles. In addition to the high and tailorable porosity, benzoxazines have several properties that make them good for numerous applications of the carbonized forms, including electrodes, batteries, gas adsorbents, catalysts, shielding materials, and intumescent coatings, among others. Extreme thermal and electrical stability also allows benzoxazines to be used in harsher conditions, such as in aerospace applications.
Keywords: advanced carbon material; benzoxazine; polybenzoxazine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


































References
-
- Osawa E., Kroto H.W., Fowler P.W., Wasserman E. The evolution of the football structure for the C60 molecule: A retrospective. The Fullerenes. 1993;2010:1–8. doi: 10.1017/cbo9780511622946.001. - DOI
-
- Kroto H.W., Heath J.R., O’Brien S.C., Curl R.F., Smalley R.E. C60: Buckminsterfullerene. Nat. Cell Biol. 1985;318:162–163. doi: 10.1038/318162a0. - DOI
-
- Iijima S. Helical microtubules of graphitic carbon. Nature. 1991;354:56–58. doi: 10.1038/354056a0. - DOI
-
- Hummers W.S., Jr., Offeman R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958;80:1339. doi: 10.1021/ja01539a017. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

