The Continuum of Thyroid Disorders Related to Immune Checkpoint Inhibitors: Still Many Pending Queries
- PMID: 34771441
- PMCID: PMC8582503
- DOI: 10.3390/cancers13215277
The Continuum of Thyroid Disorders Related to Immune Checkpoint Inhibitors: Still Many Pending Queries
Abstract
Background: Until more data are available to shed light on the thyroid disorders related to immune checkpoint inhibitors (ICPi) implemented for the treatment of hematological malignancies, the decision-making is guided by pertinent data derived mostly from solid tumors.
Methods: The present review provides a comprehensive and updated overview of the thyroid disorders related to ICPi, namely to inhibitors of cytotoxic T-lymphocyte antigen 4 (CTLA-4), programmed cell death (PD) 1 (PD-1), and the ligand of the latter (PD-L1).
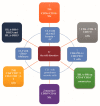
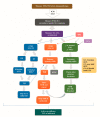
Results: With the increasing recognition of ir thyroid disorders, many outstanding issues have emerged. Ir thyroid disorders are reminiscent of, but not identical to, thyroid autoimmunity. Interclass and intraclass ICPi differences regarding thyroid immunotoxicity await interpretation. The available data concerning the predictive value of thyroid autoantibodies for the development of ir thyroid disorders are inconclusive. Mounting data indicate an association of ir thyroid disorders with ICPi efficacy, but a causative link is still lacking. The path forward is a tailored approach, entailing: (i) the validation of tumor-specific, patient-specific, and ICPi-specific predictive factors; (ii) appropriate patient selection; (iii) the uncoupling of antitumor immunity from immunotoxicity; (iv) a multidisciplinary initiative; and (v) global registry strategies.
Conclusions: Untangling and harnessing the interrelationship of immuno-oncology with endocrinology underlying the ir thyroid disorders will yield the optimal patient care.
Keywords: Graves’ disease; anti-CTLA-4 monoclonal antibodies; anti-PD-1 monoclonal antibodies; anti-PD-L1; hyperthyroidism; hypothyroidism; immune checkpoint inhibitors; immune-related adverse events; monoclonal antibodies; thyroid autoantibodies; thyrotoxicosis.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Raschi E., Mazzarella A., Antonazzo I.C., Bendinelli N., Forcesi E., Tuccori M., Moretti U., Poluzzi E., De Ponti F. Toxicities with Immune Checkpoint Inhibitors: Emerging Priorities From Disproportionality Analysis of the FDA Adverse Event Reporting System. Target Oncol. 2019;14:205–221. doi: 10.1007/s11523-019-00632-w. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

