IL-17B/RB Activation in Pancreatic Stellate Cells Promotes Pancreatic Cancer Metabolism and Growth
- PMID: 34771503
- PMCID: PMC8611647
- DOI: 10.3390/cancers13215338
IL-17B/RB Activation in Pancreatic Stellate Cells Promotes Pancreatic Cancer Metabolism and Growth
Abstract
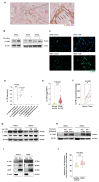
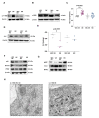
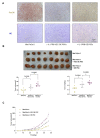
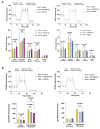
In pancreatic ductal adenocarcinoma (PDAC), the tumor stroma constitutes most of the cell mass and contributes to therapy resistance and progression. Here we show a hitherto unknown metabolic cooperation between pancreatic stellate cells (PSCs) and tumor cells through Interleukin 17B/Interleukin 17B receptor (IL-17B/IL-17RB) signaling. Tumor-derived IL-17B carrying extracellular vesicles (EVs) activated stromal PSCs and induced the expression of IL-17RB. PSCs increased oxidative phosphorylation while reducing mitochondrial turnover. PSCs activated tumor cells in a feedback loop. Tumor cells subsequently increased oxidative phosphorylation and decreased glycolysis partially via IL-6. In vivo, IL-17RB overexpression in PSCs accelerated tumor growth in a co-injection xenograft mouse model. Our results demonstrate a tumor-to-stroma feedback loop increasing tumor metabolism to accelerate tumor growth under optimal nutritional conditions.
Keywords: IL17B/RB; metabolism; pancreatic cancer; tumor microenvironment.
Conflict of interest statement
None declared. The authors declare no competing interest.
Figures




Similar articles
-
Positive feedback in Cav-1-ROS signalling in PSCs mediates metabolic coupling between PSCs and tumour cells.J Cell Mol Med. 2020 Aug;24(16):9397-9408. doi: 10.1111/jcmm.15596. Epub 2020 Jul 7. J Cell Mol Med. 2020. PMID: 32633891 Free PMC article.
-
A positive feedback loop of IL-17B-IL-17RB activates ERK/β-catenin to promote lung cancer metastasis.Cancer Lett. 2018 May 28;422:44-55. doi: 10.1016/j.canlet.2018.02.037. Epub 2018 Feb 26. Cancer Lett. 2018. PMID: 29496538
-
Galectin-3 Mediates Tumor Cell-Stroma Interactions by Activating Pancreatic Stellate Cells to Produce Cytokines via Integrin Signaling.Gastroenterology. 2018 Apr;154(5):1524-1537.e6. doi: 10.1053/j.gastro.2017.12.014. Epub 2017 Dec 21. Gastroenterology. 2018. PMID: 29274868
-
The Emerging Role of the IL-17B/IL-17RB Pathway in Cancer.Front Immunol. 2020 Apr 21;11:718. doi: 10.3389/fimmu.2020.00718. eCollection 2020. Front Immunol. 2020. PMID: 32373132 Free PMC article. Review.
-
The critical roles of activated stellate cells-mediated paracrine signaling, metabolism and onco-immunology in pancreatic ductal adenocarcinoma.Mol Cancer. 2018 Feb 19;17(1):62. doi: 10.1186/s12943-018-0815-z. Mol Cancer. 2018. PMID: 29458370 Free PMC article. Review.
Cited by
-
Pancreatic stellate cells in pancreatic cancer: as potential targets for future therapy.Front Oncol. 2023 Jun 20;13:1185093. doi: 10.3389/fonc.2023.1185093. eCollection 2023. Front Oncol. 2023. PMID: 37409257 Free PMC article. Review.
-
Role and functional mechanisms of IL‑17/IL‑17R signaling in pancreatic cancer (Review).Oncol Rep. 2024 Nov;52(5):144. doi: 10.3892/or.2024.8803. Epub 2024 Sep 2. Oncol Rep. 2024. PMID: 39219271 Free PMC article. Review.
-
Extracellular vesicle-mediated crosstalk between pancreatic cancer and stromal cells in the tumor microenvironment.J Nanobiotechnology. 2022 May 2;20(1):208. doi: 10.1186/s12951-022-01382-0. J Nanobiotechnology. 2022. PMID: 35501802 Free PMC article. Review.
-
The Role of Extracellular Vesicles in Metabolic Reprogramming of the Tumor Microenvironment.Cells. 2022 Apr 23;11(9):1433. doi: 10.3390/cells11091433. Cells. 2022. PMID: 35563739 Free PMC article. Review.
-
Prognostic value of FCER1G expression and M2 macrophage infiltration in esophageal squamous cell carcinoma.Discov Oncol. 2025 Feb 3;16(1):113. doi: 10.1007/s12672-025-01843-6. Discov Oncol. 2025. PMID: 39899137 Free PMC article.
References
-
- Latenstein A.E.J., van der Geest L.G.M., Bonsing B.A., Groot Koerkamp B., Haj Mohammad N., de Hingh I.H.J.T., de Meijer V.E., Molenaar I.Q., van Santvoort H.C., van Tienhoven G., et al. Nationwide Trends in Incidence, Treatment and Survival of Pancreatic Ductal Adenocarcinoma. Eur. J. Cancer Oxf. Engl. 1990. 2020;125:83–93. doi: 10.1016/j.ejca.2019.11.002. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

