Nicotinic Acetylcholine Receptor Subunit α7 Mediates Cigarette Smoke-Induced PD-L1 Expression in Human Bronchial Epithelial Cells
- PMID: 34771509
- PMCID: PMC8582493
- DOI: 10.3390/cancers13215345
Nicotinic Acetylcholine Receptor Subunit α7 Mediates Cigarette Smoke-Induced PD-L1 Expression in Human Bronchial Epithelial Cells
Abstract
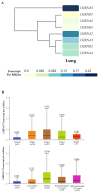
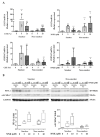
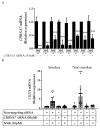
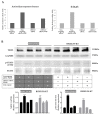
Tobacco smoking is the top risk factor for lung cancer development. Nicotine in cigarettes can induce addiction, and its derivatives become potent carcinogens after metabolic activation and activate oncogenic signaling in lung epithelial cells through their expressed nicotinic acetylcholine receptors (nAChRs). However, the effects of smoking on the tumor immune microenvironment are under investigation. In the current study, we investigated whether nicotine activation of nicotinic acetylcholine receptor subunit α7 (nAChRα7, CHRNA7) would induce PD-L1 expression in lung epithelial cells. The expression levels of nAChRα7 and PD-L1 in eight human bronchial epithelial cell (HBEC) lines were measured after treatment with cigarette smoke extract (CSE) or nicotine derivatives. The results showed that PD-L1 expression levels increased in HBECs after exposure to CSE or nicotine derivatives. This induction of PD-L1 expression could be diminished by treatment with CHRNA7 small-interfering RNA, and the relevant signaling was mediated via STAT3 phosphorylation and NRF2 expression. In summary, this study demonstrated that the well-known nicotine derivative-activated nAChRα7 could induce STAT3/NRF2 pathways and subsequently promote PD-L1 expression in normal lung epithelial cells. This information provides mechanistic insight into cigarette smoke-induced immune evasion in lung epithelial cells.
Keywords: CHRNA7; PD-L1; bronchial epithelial cells; cigarette smoke; lung cancer.
Conflict of interest statement
J.D.M. receives licensing royalties from the US National Institute of Health, and the University of Texas Southwestern Medical Center at Dallas for distribution of human tumor cells and HBEC strains. The other authors declare no conflict of interest.
Figures




Similar articles
-
Cigarette Smoke Induces Stem Cell Features of Pancreatic Cancer Cells via PAF1.Gastroenterology. 2018 Sep;155(3):892-908.e6. doi: 10.1053/j.gastro.2018.05.041. Epub 2018 Jun 2. Gastroenterology. 2018. PMID: 29864419 Free PMC article.
-
Nicotine activates cell-signaling pathways through muscle-type and neuronal nicotinic acetylcholine receptors in non-small cell lung cancer cells.Pulm Pharmacol Ther. 2007;20(6):629-41. doi: 10.1016/j.pupt.2006.07.001. Epub 2006 Aug 18. Pulm Pharmacol Ther. 2007. PMID: 17015027
-
Nicotine-Mediated Regulation of Nicotinic Acetylcholine Receptors in Non-Small Cell Lung Adenocarcinoma by E2F1 and STAT1 Transcription Factors.PLoS One. 2016 May 26;11(5):e0156451. doi: 10.1371/journal.pone.0156451. eCollection 2016. PLoS One. 2016. PMID: 27228072 Free PMC article.
-
α7 nicotinic acetylcholine receptor subunit in angiogenesis and epithelial to mesenchymal transition.Curr Drug Targets. 2012 May;13(5):671-9. doi: 10.2174/138945012800398847. Curr Drug Targets. 2012. PMID: 22300034 Review.
-
The potential role of nicotine in breast cancer initiation, development, angiogenesis, invasion, metastasis, and resistance to therapy.Breast Cancer. 2022 Sep;29(5):778-789. doi: 10.1007/s12282-022-01369-7. Epub 2022 May 18. Breast Cancer. 2022. PMID: 35583594 Review.
Cited by
-
Peptide Mimicking Loop II of the Human Epithelial Protein SLURP-2 Enhances the Viability and Migration of Skin Keratinocytes.Acta Naturae. 2024 Oct-Dec;16(4):86-94. doi: 10.32607/actanaturae.27494. Acta Naturae. 2024. PMID: 39877008 Free PMC article.
-
Tobacco smoke exposure is a driver of altered oxidative stress response and immunity in head and neck cancer.J Transl Med. 2025 Apr 5;23(1):403. doi: 10.1186/s12967-025-06258-z. J Transl Med. 2025. PMID: 40188338 Free PMC article.
-
PD-L1 expression in patients with non-small-cell lung cancer is associated with sex and genetic alterations: A retrospective study within the Caucasian population.Thorac Cancer. 2024 Jul;15(20):1598-1606. doi: 10.1111/1759-7714.15336. Epub 2024 Jun 11. Thorac Cancer. 2024. PMID: 38860475 Free PMC article.
-
An integral perspective of canonical cigarette and e-cigarette-related cardiovascular toxicity based on the adverse outcome pathway framework.J Adv Res. 2023 Jun;48:227-257. doi: 10.1016/j.jare.2022.08.012. Epub 2022 Aug 21. J Adv Res. 2023. PMID: 35998874 Free PMC article. Review.
-
The Role of the Acetylcholine System in Common Respiratory Diseases and COVID-19.Molecules. 2023 Jan 23;28(3):1139. doi: 10.3390/molecules28031139. Molecules. 2023. PMID: 36770805 Free PMC article. Review.
References
-
- Le Marchand L., Derby K.S., Murphy S.E., Hecht S.S., Hatsukami D., Carmella S.G., Tiirikainen M., Wang H. Smokers with the CHRNA lung cancer-associated variants are exposed to higher levels of nicotine equivalents and a carcinogenic tobacco-specific nitrosamine. Cancer Res. 2008;68:9137–9140. doi: 10.1158/0008-5472.CAN-08-2271. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

