Significant Inter- and Intralaboratory Variation in Gleason Grading of Prostate Cancer: A Nationwide Study of 35,258 Patients in The Netherlands
- PMID: 34771542
- PMCID: PMC8582481
- DOI: 10.3390/cancers13215378
Significant Inter- and Intralaboratory Variation in Gleason Grading of Prostate Cancer: A Nationwide Study of 35,258 Patients in The Netherlands
Abstract
Purpose: Our aim was to analyze grading variation between pathology laboratories and between pathologists within individual laboratories using nationwide real-life data.
Methods: We retrieved synoptic (n = 13,397) and narrative (n = 29,377) needle biopsy reports from the Dutch Pathology Registry and prostate-specific antigen values from The Netherlands Cancer Registration for prostate cancer patients diagnosed between January 2017 and December 2019. We determined laboratory-specific proportions per histologic grade and unadjusted odds ratios (ORs) for International Society of Urological Pathologists Grades 1 vs. 2-5 for 40 laboratories due to treatment implications for higher grades. Pathologist-specific proportions were determined for 21 laboratories that consented to this part of analysis. The synoptic reports of 21 laboratories were used for analysis of case-mix correction for PSA, age, year of diagnosis, number of biopsies and positive cores.
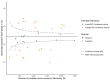
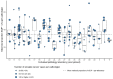
Results: A total of 38,321 reports of 35,258 patients were included. Grade 1 ranged between 19.7% and 44.3% per laboratory (national mean = 34.1%). Out of 40 laboratories, 22 (55%) reported a significantly deviant OR, ranging from 0.48 (95% confidence interval (CI) 0.39-0.59) to 1.54 (CI 1.22-1.93). Case-mix correction was performed for 10,294 reports, altering the status of 3/21 (14%) laboratories, but increasing the observed variation (20.8% vs. 17.7%). Within 15/21 (71%) of laboratories, significant inter-pathologist variation existed.
Conclusion: Substantial variation in prostate cancer grading was observed between and within Dutch pathology laboratories. Case-mix correction did not explain the variation. Better standardization of prostate cancer grading is warranted to optimize and harmonize treatment.
Keywords: Gleason grading; interlaboratory variation; pathology; prostate cancer.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
-
- Cijfers Over Kanker Netherlands Cancer Registry supplied by IKNL. [(accessed on 25 May 2020)]. Published 2020. Available online: www.cijfersoverkanker.nl.
-
- Joniau S., Briganti A., Gontero P., Gandaglia G., Tosco L., Fieuws S., Tombal B., Marchioro G., Walz J., Kneitz B., et al. Stratification of High-risk Prostate Cancer into Prognostic Categories: A European Multi-institutional Study. Eur. Urol. 2015;67:157–164. doi: 10.1016/j.eururo.2014.01.020. - DOI - PubMed
-
- Mottet N., Bellmunt J., Bolla M., Briers E., Cumberbatch M.G., De Santis M., Fossati N., Gross T., Henry A.M., Joniau S., et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2017;71:618–629. doi: 10.1016/j.eururo.2016.08.003. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

