Enhancement of NK Cell Antitumor Effector Functions Using a Bispecific Single Domain Antibody Targeting CD16 and the Epidermal Growth Factor Receptor
- PMID: 34771609
- PMCID: PMC8582566
- DOI: 10.3390/cancers13215446
Enhancement of NK Cell Antitumor Effector Functions Using a Bispecific Single Domain Antibody Targeting CD16 and the Epidermal Growth Factor Receptor
Abstract
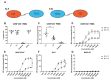
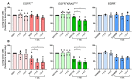
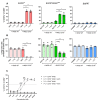
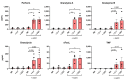
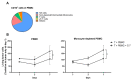
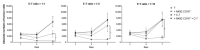
The ability to kill tumor cells while maintaining an acceptable safety profile makes Natural Killer (NK) cells promising assets for cancer therapy. Strategies to enhance the preferential accumulation and activation of NK cells in the tumor microenvironment can be expected to increase the efficacy of NK cell-based therapies. In this study, we show binding of a novel bispecific single domain antibody (VHH) to both CD16 (FcRγIII) on NK cells and the epidermal growth factor receptor (EGFR) on tumor cells of epithelial origin. The bispecific VHH triggered CD16- and EGFR-dependent activation of NK cells and subsequent lysis of tumor cells, regardless of the KRAS mutational status of the tumor. Enhancement of NK cell activation by the bispecific VHH was also observed when NK cells of colorectal cancer (CRC) patients were co-cultured with EGFR expressing tumor cells. Finally, higher levels of cytotoxicity were found against patient-derived metastatic CRC cells in the presence of the bispecific VHH and autologous peripheral blood mononuclear cells or allogeneic CD16 expressing NK cells. The anticancer activity of CD16-EGFR bispecific VHHs reported here merits further exploration to assess its potential therapeutic activity either alone or in combination with adoptive NK cell-based therapeutic approaches.
Keywords: CD16; EGFR; NK cells; bispecific VHH; single domain antibodies.
Conflict of interest statement
Jan Spanholtz is chief scientific officer at Glycostem BV and Hans. J. van der Vliet is chief scientific officer at Lava Therapeutics NV. Tanja de Gruijl is scientific advisor to Lava Therapeutics. Amanda van Vliet is an employee of Glycostem BV. No other conflict of interest is reported.
Figures


Similar articles
-
Incorporation of a Novel CD16-Specific Single-Domain Antibody into Multispecific Natural Killer Cell Engagers With Potent ADCC.Mol Pharm. 2021 Jun 7;18(6):2375-2384. doi: 10.1021/acs.molpharmaceut.1c00208. Epub 2021 May 17. Mol Pharm. 2021. PMID: 33999642
-
Impact of antibody architecture and paratope valency on effector functions of bispecific NKp30 x EGFR natural killer cell engagers.MAbs. 2024 Jan-Dec;16(1):2315640. doi: 10.1080/19420862.2024.2315640. Epub 2024 Feb 19. MAbs. 2024. PMID: 38372053 Free PMC article.
-
Tribody [(HER2)2xCD16] Is More Effective Than Trastuzumab in Enhancing γδ T Cell and Natural Killer Cell Cytotoxicity Against HER2-Expressing Cancer Cells.Front Immunol. 2018 Apr 19;9:814. doi: 10.3389/fimmu.2018.00814. eCollection 2018. Front Immunol. 2018. PMID: 29725336 Free PMC article.
-
Overcoming Resistance to Natural Killer Cell Based Immunotherapies for Solid Tumors.Front Oncol. 2019 Feb 11;9:51. doi: 10.3389/fonc.2019.00051. eCollection 2019. Front Oncol. 2019. PMID: 30805309 Free PMC article. Review.
-
Harnessing CD16-Mediated NK Cell Functions to Enhance Therapeutic Efficacy of Tumor-Targeting mAbs.Cancers (Basel). 2021 May 20;13(10):2500. doi: 10.3390/cancers13102500. Cancers (Basel). 2021. PMID: 34065399 Free PMC article. Review.
Cited by
-
Allogeneic NK cells induce monocyte-to-dendritic cell conversion, control tumor growth, and trigger a pro-inflammatory shift in patient-derived cultures of primary and metastatic colorectal cancer.J Immunother Cancer. 2023 Dec 6;11(12):e007554. doi: 10.1136/jitc-2023-007554. J Immunother Cancer. 2023. PMID: 38056896 Free PMC article.
-
Novel sACE2-Anti-CD16VHH Fusion Protein Surreptitiously Inhibits SARS-CoV-2 Variant Spike Proteins and Macrophage Cytokines, and Activates Natural Killer Cell Cytotoxicity.Vaccines (Basel). 2025 Feb 17;13(2):199. doi: 10.3390/vaccines13020199. Vaccines (Basel). 2025. PMID: 40006745 Free PMC article.
-
Nuclear epidermal growth factor receptor as a therapeutic target.Explor Target Antitumor Ther. 2023;4(4):616-629. doi: 10.37349/etat.2023.00156. Epub 2023 Aug 30. Explor Target Antitumor Ther. 2023. PMID: 37720348 Free PMC article. Review.
-
Harnessing the Power of NK Cell Receptor Engineering as a New Prospect in Cancer Immunotherapy.Pharmaceutics. 2024 Aug 29;16(9):1143. doi: 10.3390/pharmaceutics16091143. Pharmaceutics. 2024. PMID: 39339180 Free PMC article. Review.
-
Research Progress and Applications of Multivalent, Multispecific and Modified Nanobodies for Disease Treatment.Front Immunol. 2022 Jan 18;12:838082. doi: 10.3389/fimmu.2021.838082. eCollection 2021. Front Immunol. 2022. PMID: 35116045 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

