The Neglected Liaison: Targeting Cancer Cell Metabolic Reprogramming Modifies the Composition of Non-Malignant Populations of the Tumor Microenvironment
- PMID: 34771610
- PMCID: PMC8582418
- DOI: 10.3390/cancers13215447
The Neglected Liaison: Targeting Cancer Cell Metabolic Reprogramming Modifies the Composition of Non-Malignant Populations of the Tumor Microenvironment
Abstract
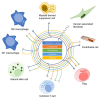
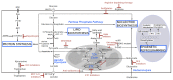
Metabolic reprogramming is a well-known hallmark of cancer, whereby the development of drugs that target cancer cell metabolism is gaining momentum. However, when establishing preclinical studies and clinical trials, it is often neglected that a tumor mass is a complex system in which cancer cells coexist and interact with several types of microenvironment populations, including endothelial cells, fibroblasts and immune cells. We are just starting to understand how such populations are affected by the metabolic changes occurring in a transformed cell and little is known about the impact of metabolism-targeting drugs on the non-malignant tumor components. Here we provide a general overview of the links between cancer cell metabolism and tumor microenvironment (TME), particularly focusing on the emerging literature reporting TME-specific effects of metabolic therapies.
Keywords: cancer metabolism; cancer-associated fibroblasts; metabolic reprogramming; tumor microenvironment; tumor-associated macrophages.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Metabolic Cooperation and Competition in the Tumor Microenvironment: Implications for Therapy.Front Oncol. 2017 Apr 12;7:68. doi: 10.3389/fonc.2017.00068. eCollection 2017. Front Oncol. 2017. PMID: 28447025 Free PMC article. Review.
-
Metabolic reprogramming of T regulatory cells in the hypoxic tumor microenvironment.Cancer Immunol Immunother. 2021 Aug;70(8):2103-2121. doi: 10.1007/s00262-020-02842-y. Epub 2021 Feb 3. Cancer Immunol Immunother. 2021. PMID: 33532902 Free PMC article. Review.
-
Metabolism within the tumor microenvironment and its implication on cancer progression: An ongoing therapeutic target.Med Res Rev. 2019 Jan;39(1):70-113. doi: 10.1002/med.21511. Epub 2018 May 22. Med Res Rev. 2019. PMID: 29785785 Review.
-
Tumor Microenvironment and Nitric Oxide: Concepts and Mechanisms.Adv Exp Med Biol. 2020;1277:143-158. doi: 10.1007/978-3-030-50224-9_10. Adv Exp Med Biol. 2020. PMID: 33119871
-
Metabolic Switch in the Tumor Microenvironment Determines Immune Responses to Anti-cancer Therapy.Front Oncol. 2018 Aug 13;8:284. doi: 10.3389/fonc.2018.00284. eCollection 2018. Front Oncol. 2018. PMID: 30151352 Free PMC article. Review.
Cited by
-
Refining the optimal CAF cluster marker for predicting TME-dependent survival expectancy and treatment benefits in NSCLC patients.Sci Rep. 2024 Jul 21;14(1):16766. doi: 10.1038/s41598-024-55375-0. Sci Rep. 2024. PMID: 39034310 Free PMC article.
-
GLS1 promotes lipid metabolism in hepatocellular carcinoma by regulating the PI3K/AKT/mTORC1 signaling pathway through SREBP-1.Am J Transl Res. 2025 Apr 15;17(4):2527-2540. doi: 10.62347/ZTGP5030. eCollection 2025. Am J Transl Res. 2025. PMID: 40385002 Free PMC article.
References
-
- Martínez-Reyes I., Diebold L.P., Kong H., Schieber M., Huang H., Hensley C.T., Mehta M.M., Wang T., Santos J.H., Woychik R., et al. TCA Cycle and Mitochondrial Membrane Potential Are Necessary for Diverse Biological Functions. Mol. Cell. 2016;61:199–209. doi: 10.1016/j.molcel.2015.12.002. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

