First-Line Treatment of Metastatic Clear Cell Renal Cell Carcinoma: What Are the Most Appropriate Combination Therapies?
- PMID: 34771710
- PMCID: PMC8583335
- DOI: 10.3390/cancers13215548
First-Line Treatment of Metastatic Clear Cell Renal Cell Carcinoma: What Are the Most Appropriate Combination Therapies?
Abstract
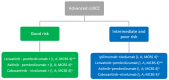
The development of antiangiogenic treatments, followed by immune checkpoint inhibitors (ICI), has significantly changed the management of metastatic clear cell renal cell cancer. Several phase III trials show the superiority of combination therapy, dual immunotherapy (ICI-ICI) or ICI plus tyrosine kinase inhibitors (TKI) of the vascular endothelium growth factor (VEGF) over sunitinib monotherapy. The question is therefore what is the best combination for a given patient? A strategy based on the International Metastatic Database Consortium (IMDC) classification is currently recommended with pembrolizumab + axitinib, cabozantinib + nivolumab, and lenvatinib + pembrolizumab (for all patients) or nivolumab + ipilimumab (for patients with intermediate or poor risk), which are the first-line treatment standards of care. However, several issues remain unresolved and require further investigation, such as the PD-L1 status, the relevance of possible options based on the patient's profile, and consideration of second-line and subsequent treatments.
Keywords: combinations; first-line treatment; immunotherapy; metastatic clear cell renal cell carcinoma; tyrosine kinase inhibitors.
Conflict of interest statement
Yann-Alexandre Vano has received honoraria for advisory board from Bristol-Myers Squibb, Pfizer, Novartis, Ipsen, Merck, MSD, Janssen, Sanofi, Astellas Pharma, and Roche, and travel expenses/accommodations from Bristol-Myers Squibb, Pfizer, MSD, and Roche. Sylvain Ladoire has received research funding from Novartis, honoraria for consultancy from Lilly, Pfizer, Novartis, Bristol-Myers Squibb, Astellas Pharma, Roche, Ipsen, Janssen Oncology, and Sanofi, and travel expenses/accommodations from Pfizer, Novartis, AstraZeneca, Sanofi, Astellas Pharma, Janssen Oncology, Ipsen, Bristol-Myers Squibb. Reza Elaidi has received honoraria for advisory board from Pfizer. Slimane Dermeche has received honoraria for advisory board from Pfizer. Jean-Christophe Eymard has received honoraria for consultancy from Bristol-Myers Squibb, Pfizer, Ipsen, Novartis, Janssen, Astellas and Sanofi, and travel expenses/accommodations from Bristol-Myers Squibb and Pfizer. Sabrina Falkowski has received honoraria for advisory board from Bristol-Myers Squibb, Pfizer, Sanofi, MSD, Janssen, AstraZeneca and Astellas, and travel expenses/accommodations from Pfizer, Sanofi, MSD, and Janssen. Marine Gross-Goupil has received honoraria for advisory board/consultancy from Bristol-Myers Squibb, MSD, Pfizer, Merck, Ipsen, and Novartis, and as a speaker from Roche and AstraZeneca, and travel expenses/accommodations from Bristol-Myers Squibb, Pfizer, and Ipsen. Gabriel Malouf has received honoraria for consultancy from Pfizer, Bristol-Myers Squibb, Astellas Pharma, and Ipsen. Berangere Narciso has received honoraria for advisory board from Janssen, Ipsen, Bristol-Myers Squibb and MSD. Christophe Sajous has received honoraria for advisory board from Pfizer, for consultancy from AstraZeneca, and as a speaker from Lilly. Sophie Tartas has received honoraria for advisory board from Pfizer. Eric Voog has received honoraria for advisory board from Pfizer. Alain Ravaud has received honoraria for advisory board/consultancy from Bristol-Myers Squibb, Pfizer, Novartis, Ipsen, Merck, MSD, AstraZeneca and Roche, and travel expenses/accommodations from Pfizer, Novartis, Bristol-Myers Squibb, Astra Zeneca, Ipsen, and MSD.
Figures
References
-
- Lalani A.-K.A., McGregor B.A., Albiges L., Choueiri T.K., Motzer R., Powles T., Wood C., Bex A. Systemic Treatment of Metastatic Clear Cell Renal Cell Carcinoma in 2018: Current Paradigms, Use of Immunotherapy, and Future Directions. Eur. Urol. 2019;75:100–110. doi: 10.1016/j.eururo.2018.10.010. - DOI - PubMed
-
- Motzer R.J., Tannir N.M., McDermott D.F., Arén Frontera O., Melichar B., Choueiri T.K., Plimack E.R., Barthélémy P., Porta C., George S., et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018;378:1277–1290. doi: 10.1056/NEJMoa1712126. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

