Comparison of Efficacy in Patients with Metastatic Melanoma Treated with Ipilimumab and Nivolumab Who Did or Did Not Discontinue Treatment Due to Immune-Related Adverse Events: A Real-World Data Study
- PMID: 34771712
- PMCID: PMC8583558
- DOI: 10.3390/cancers13215550
Comparison of Efficacy in Patients with Metastatic Melanoma Treated with Ipilimumab and Nivolumab Who Did or Did Not Discontinue Treatment Due to Immune-Related Adverse Events: A Real-World Data Study
Abstract
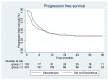
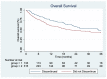
Immune-related adverse events (irAEs) are very prevalent when treating patients with ipilimumab and nivolumab in combination, and 30-40% of patients discontinue the treatment for this reason. It is of high clinical relevance to investigate the consequences of discontinuing the treatment early since combination therapy with ipilimumab and nivolumab is the first line of treatment for many patients with metastatic melanoma. In this follow-up study, with real-world data from the nationwide DAMMED database, we investigated whether there was a difference in progression-free survival (PFS) and overall survival (OS) for patients who discontinued or did not discontinue treatment within the first four doses of treatment due to irAEs. In total, 448 patients were treated with ipilimumab and nivolumab. Of these, 133 patients discontinued due to irAEs in the induction phase. Using the Cox proportional hazards model, there was no significant difference in PFS when comparing the group that discontinued with the group that did not discontinue. The group that discontinued had a significantly longer OS than the group that received the full length of treatment. Therefore, we conclude that there is no significant negative impact on efficacy for patients who discontinue due to irAEs in the induction phase of combination immunotherapy for metastatic melanoma.
Keywords: DAMMED; immune-related adverse events; immunotherapy; ipilimumab; melanoma; nivolumab.
Conflict of interest statement
Inge Marie Svane has received honoraria for consultancies and lectures from Novartis, Roche, Merck and Bristol-Myers Squibb; a restricted research grant from Novartis; and financial support for attending symposia from Bristol-Myers Squibb, Merck, Novartis, Pfizer and Roche. Marco Donia has for the past two years received honoraria for lectures from Roche, and institutional research support from Bristol Myers Squibb. Eva Ellebaek has received honoraria from BMS, Pierre Fabre, and Novartis for consultancies and lectures, and travel/conference expenses from MSD.
Figures
References
-
- Rastrelli M., Tropea S., Rossi C.R., Alaibac M. Melanoma: Epidemiology, Risk Factors, Pathogenesis, Diagnosis and Classification. Vivo. 2014;28:1005–1011. - PubMed
-
- WHO Globocan Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. [(accessed on 2 November 2021)];Int. Agency Res. Cancer. 2012 Available online: https://gco.iarc.fr/today/home.
-
- Lanoy E. Epidemiology, risk factor and screening for melanoma and other skin cancers. Rev. Prat. 2014;64:31–36. - PubMed
-
- Hodi F.S., Chiarion-Sileni V., Gonzalez R., Grob J.J., Rutkowski P., Cowey C.L., Lao C.D., Schadendorf D., Wagstaff J., Dummer R., et al. Nivolumab plus Ipilimumab or Nivolumab Alone versus Ipilimumab Alone in Advanced Melanoma (CheckMate 067): 4-Year Outcomes of a Multicentre, Randomised, Phase 3 Trial. Lancet Oncol. 2018;19:1480–1492. doi: 10.1016/S1470-2045(18)30700-9. - DOI - PubMed
LinkOut - more resources
Full Text Sources