Radioimmunotherapy in Oncology: Overview of the Last Decade Clinical Trials
- PMID: 34771732
- PMCID: PMC8583425
- DOI: 10.3390/cancers13215570
Radioimmunotherapy in Oncology: Overview of the Last Decade Clinical Trials
Abstract
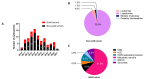
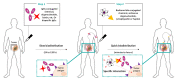
The specific irradiation of tumors with selective radiolabeled antibodies constitutes an attractive therapeutic approach. Consequent preclinical research has been conducted by both biologists to identify pertinent targets and to select corresponding antibodies (mAb) and by radiochemists to radiolabel mAbs. These numerous preclinical investigations have ascertained the therapeutic interest of radioimmunotherapy (RIT) protocols in mice models. Here, we summarize the clinical studies that have been performed the last decade, including clinical trials (phases I, II, and III), prospective and retrospective studies, and cases series. We thereby reported 92 clinical studies. Among them, 62 concern the treatment of hematological malignancies, and 30 concern solid tumors. For hematologic diseases, the analysis was complex due to the high discrepancy of therapeutic strategies (first-line therapy, consolidation, stem cell transplantation conditioning) as well as the high variety of malignancies that were treated. The clinical studies from the last decade failed to expand anti-CD20 RIT indications but confirmed that RIT using radiolabeled anti-CD20 remains a pertinent choice for patients with relapse follicular lymphomas. For solid tumors, the positive benefit of RIT is more mitigated, apart for few malignancies that can be treated locally. Clinical trials also demonstrated the potential of some antibody formats, such as F(ab')2, which has already been approved by the China State FDA under the trend name Licartin®. Despite disparate results, mAb fragments are an interesting prospect for the improvement of RIT efficiency as well as for pretargeted strategies that delay the injection of radioactive treatments from the mAb ones.
Keywords: PRIT; RIT; antibody fragments; hematologic cancers; radionuclides; solid cancers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

