ERAS, a Member of the Ras Superfamily, Acts as an Oncoprotein in the Mammary Gland
- PMID: 34771750
- PMCID: PMC8582886
- DOI: 10.3390/cancers13215588
ERAS, a Member of the Ras Superfamily, Acts as an Oncoprotein in the Mammary Gland
Abstract
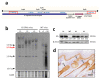
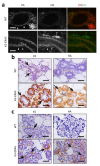
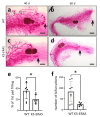
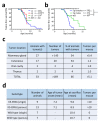
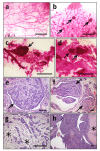
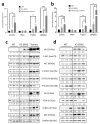
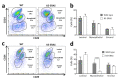
ERAS is a relatively uncharacterized gene of the Ras superfamily. It is expressed in ES cells and in the first stages of embryonic development; later on, it is silenced in the majority of cell types and tissues. Although there are several reports showing ERAS expression in tumoral cell lines and human tumor samples, it is unknown if ERAS deregulated expression is enough to drive tumor development. In this report, we have generated transgenic mice expressing ERAS in myoepithelial basal cells of the mammary gland and in basal cells of stratified epithelia. In spite of the low level of ERAS expression, these transgenic mice showed phenotypic alterations resembling overgrowth syndromes caused by the activation of the AKT-PI3K pathway. In addition, their mammary glands present developmental and functional disabilities accompanied by morphological and biochemical alterations in the myoepithelial cells. These mice suffer from tumoral transformation in the mammary glands with high incidence. These mammary tumors resemble, both histologically and by the expression of differentiation markers, malignant adenomyoepitheliomas. In sum, our results highlight the importance of ERAS silencing in adult tissues and define a truly oncogenic role for ERAS in mammary gland cells when inappropriately expressed.
Keywords: ERAS; Ras pathway; adenomyoepithelioma; breast cancer; transgenic mice.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



Similar articles
-
Insertional mutagenesis in a HER2-positive breast cancer model reveals ERAS as a driver of cancer and therapy resistance.Oncogene. 2018 Mar;37(12):1594-1609. doi: 10.1038/s41388-017-0031-0. Epub 2018 Jan 12. Oncogene. 2018. PMID: 29326437 Free PMC article.
-
The Ras-related gene ERAS is involved in human and murine breast cancer.Sci Rep. 2018 Aug 29;8(1):13038. doi: 10.1038/s41598-018-31326-4. Sci Rep. 2018. PMID: 30158566 Free PMC article.
-
The transcription factor ATF3 acts as an oncogene in mouse mammary tumorigenesis.BMC Cancer. 2008 Sep 22;8:268. doi: 10.1186/1471-2407-8-268. BMC Cancer. 2008. PMID: 18808719 Free PMC article.
-
Stem cells in mammary gland differentiation and cancer.J Cell Sci Suppl. 1988;10:95-114. doi: 10.1242/jcs.1988.supplement_10.8. J Cell Sci Suppl. 1988. PMID: 3077945 Review.
-
The myoepithelial cell: its role in normal mammary glands and breast cancer.Folia Morphol (Warsz). 2010 Feb;69(1):1-14. Folia Morphol (Warsz). 2010. PMID: 20235044 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

