Effects of the Manufacturing Methods on the Mechanical Properties of a Medical-Grade Copolymer Poly(L-lactide-co-D,L-lactide) and Poly(L-lactide-co-ε-caprolactone) Blend
- PMID: 34771906
- PMCID: PMC8585199
- DOI: 10.3390/ma14216381
Effects of the Manufacturing Methods on the Mechanical Properties of a Medical-Grade Copolymer Poly(L-lactide-co-D,L-lactide) and Poly(L-lactide-co-ε-caprolactone) Blend
Abstract
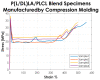
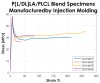
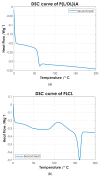
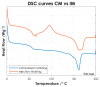
Biocompatible and biodegradable polymers represent the future in the manufacturing of medical implantable solutions. As of today, these are generally manufactured with metallic components which cannot be naturally absorbed within the human body. This requires performing an additional surgical procedure to remove the remnants after complete rehabilitation or to leave the devices in situ indefinitely. Nevertheless, the biomaterials used for this purpose must satisfy well-defined mechanical requirements. These are difficult to ascertain at the design phase since they depend not only on their physicochemical properties but also on the specific manufacturing methods used for the target application. Therefore, this research was focused on establishing the effects of the manufacturing methods on both the mechanical properties and the thermal behavior of a medical-grade copolymer blend. Specifically, Injection and Compression Molding were considered. A Poly(L-lactide-co-D,L-lactide)/Poly(L-lactide-co-ε-caprolactone) blend was considered for this investigation, with a ratio of 50/50 (w/w), aimed at the manufacturing of implantable devices for tendon repair. Interesting results were obtained.
Keywords: biodegradable; caprolactone; injection and compression molding; lactide; mechanical properties; polymer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Nardo T., Chiono V., Gentile P., Tabrizian M., Ciardelli G. Poly(DL-lactide-co-ε-caprolactone) and poly(DL-lactide-co-glycolide) blends for biomedical application: Physical properties, cell compatibility, and in vitro degradation behavior. Int. J. Polym. Mater. Polym. Biomater. 2016;65:741–750. doi: 10.1080/00914037.2016.1163566. - DOI
-
- Navarro-Baena I., Sessini V., Dominici F., Torre L., Kenny J.M., Peponi L. Design of biodegradable blends based on PLA and PCL: From morphological, thermal and mechanical studies to shape memory behavior. Polym. Degrad. Stab. 2016;132:97–108. doi: 10.1016/j.polymdegradstab.2016.03.037. - DOI
-
- Evonick Biodegradable Material Solutions to Enhance the Safety, Biocompatibility and Performance of Medical Implants. [(accessed on 21 October 2021)]. Available online: https://healthcare.evonik.com/en/medical-devices/biodegradable-materials.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

