Route of infectious bronchitis virus vaccination determines the type and magnitude of immune responses in table egg laying hens
- PMID: 34772449
- PMCID: PMC8587502
- DOI: 10.1186/s13567-021-01008-7
Route of infectious bronchitis virus vaccination determines the type and magnitude of immune responses in table egg laying hens
Abstract
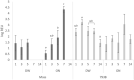
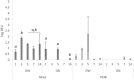
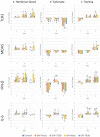
Chicken immune responses to infectious bronchitis virus (IBV) vaccination can depend on route of administration, vaccine strain and bird age. Typically for layer chickens, IBV vaccinations are administered by spray in the hatchery at day-old and boosted at intervals with live vaccines via drinking water (DW). Knowledge of live attenuated IBV vaccine virus kinetics and the immune response in egg-laying hens is exceptionally limited. Here, we demonstrated dissemination of vaccine viruses and differences in hen innate, mucosal, cellular and humoral immune responses following vaccination with Massachusetts or 793B strains, administered by DW or oculonasal (ON) routes. Detection of IBV in the Mass-vaccinated groups was greater during early time-points, however, 793B was detected more frequently at later timepoints. Viral RNA loads in the Harderian gland and turbinate tissues were significantly higher for ON-Mass compared to all other vaccinated groups. Lachrymal fluid IgY levels were significantly greater than the control at 14 days post-vaccination (dpv) for both vaccine serotypes, and IgA mRNA levels were significantly greater in ON-vaccinated groups compared to DW-vaccinated groups, demonstrating robust mucosal immune responses. Cell mediated immune gene transcripts (CD8-α and CD8-β) were up-regulated in turbinate and trachea tissues. For both vaccines, dissemination and vaccine virus clearance was slower when given by DW compared to the ON route. For ON administration, both vaccines induced comparable levels of mucosal immunity. The Mass vaccine induced cellular immunity to similar levels regardless of vaccination method. When given either by ON or DW, 793B vaccination induced significantly higher levels of humoral immunity.
Keywords: Avian coronavirus; cellular and humoral immunity; gene transcription; infectious bronchitis virus; innate; layer chicken; mucosal; vaccination.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- Cavanagh D. Coronavirus avian infectious bronchitis virus. Vet Res. 2007;38:281–297. - PubMed
-
- Cavanagh D, Gelb J. Infectious bronchitis. In: Swayne D, editor. Diseases of poultry. Ames: Blackwell Publishing; 2008.
-
- Box P, Beresford A, Roberts B. Protection of laying hens against infectious bronchitis with inactivated emulsion vaccines. Vet Rec. 1980;106:264–268. - PubMed
-
- Cook JK. Recovery of infectious bronchitis virus from eggs and chicks produced by experimentally inoculated hens. J Comp Pathol. 1971;81:203–211. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

