Csi-let-7a-5p delivered by extracellular vesicles from a liver fluke activates M1-like macrophages and exacerbates biliary injuries
- PMID: 34772807
- PMCID: PMC8609646
- DOI: 10.1073/pnas.2102206118
Csi-let-7a-5p delivered by extracellular vesicles from a liver fluke activates M1-like macrophages and exacerbates biliary injuries
Abstract
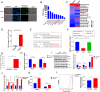
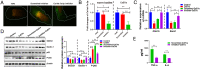
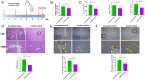
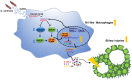
Chronic infection with liver flukes (such as Clonorchis sinensis) can induce severe biliary injuries, which can cause cholangitis, biliary fibrosis, and even cholangiocarcinoma. The release of extracellular vesicles by C. sinensis (CsEVs) is of importance in the long-distance communication between the hosts and worms. However, the biological effects of EVs from liver fluke on biliary injuries and the underlying molecular mechanisms remain poorly characterized. In the present study, we found that CsEVs induced M1-like activation. In addition, the mice that were administrated with CsEVs showed severe biliary injuries associated with remarkable activation of M1-like macrophages. We further characterized the signatures of miRNAs packaged in CsEVs and identified a miRNA Csi-let-7a-5p, which was highly enriched. Further study showed that Csi-let-7a-5p facilitated the activation of M1-like macrophages by targeting Socs1 and Clec7a; however, CsEVs with silencing Csi-let-7a-5p showed a decrease in proinflammatory responses and biliary injuries, which involved in the Socs1- and Clec7a-regulated NF-κB signaling pathway. Our study demonstrates that Csi-let-7a-5p delivered by CsEVs plays a critical role in the activation of M1-like macrophages and contributes to the biliary injuries by targeting the Socs1- and Clec7a-mediated NF-κB signaling pathway, which indicates a mechanism contributing to biliary injuries caused by fluke infection. However, molecules other than Csi-let-7a-5p from CsEVs that may also promote M1-like polarization and exacerbate biliary injuries are not excluded.
Keywords: Clonorchis sinensis; Csi-let-7a-5p; M1 macrophage; biliary injuries; extracellular vesicles.
Conflict of interest statement
The authors declare no competing interest.
Figures




References
-
- Qian M. B., Utzinger J., Keiser J., Zhou X. N., Clonorchiasis. Lancet 387, 800–810 (2016). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

