Interferon mediated prophylactic protection against respiratory viruses conferred by a prototype live attenuated influenza virus vaccine lacking non-structural protein 1
- PMID: 34773048
- PMCID: PMC8589955
- DOI: 10.1038/s41598-021-01780-8
Interferon mediated prophylactic protection against respiratory viruses conferred by a prototype live attenuated influenza virus vaccine lacking non-structural protein 1
Abstract
The influenza A non-structural protein 1 (NS1) is known for its ability to hinder the synthesis of type I interferon (IFN) during viral infection. Influenza viruses lacking NS1 (ΔNS1) are under clinical development as live attenuated human influenza virus vaccines and induce potent influenza virus-specific humoral and cellular adaptive immune responses. Attenuation of ΔNS1 influenza viruses is due to their high IFN inducing properties, that limit their replication in vivo. This study demonstrates that pre-treatment with a ΔNS1 virus results in an antiviral state which prevents subsequent replication of homologous and heterologous viruses, preventing disease from virus respiratory pathogens, including SARS-CoV-2. Our studies suggest that ΔNS1 influenza viruses could be used for the prophylaxis of influenza, SARS-CoV-2 and other human respiratory viral infections, and that an influenza virus vaccine based on ΔNS1 live attenuated viruses would confer broad protection against influenza virus infection from the moment of administration, first by non-specific innate immune induction, followed by specific adaptive immunity.
© 2021. The Author(s).
Conflict of interest statement
AG–S and PP are inventors in patents owned by the Icahn School of Medicine and licensed to Vivaldi Biosciences concerning the use of NS1 deficient viruses as human vaccines and to BI Vetmedica on the use of NS1 deficient viruses as veterinarian vaccines. The García-Sastre Laboratory has received research support from Pfizer, Senhwa Biosciences, 7Hills Pharma, Pharmamar, Blade Therapeutics, Avimex, Accurius, Dynavax, Kenall Manufacturing, ImmunityBio and Nanocomposix; and A.G.-S. has consulting agreements for the following companies involving cash and/or stock: Vivaldi Biosciences, Pagoda, Contrafect, Vaxalto, Accurius, 7Hills. The rest of the authors have no conflicts to declare.
Figures

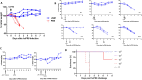
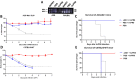
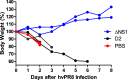

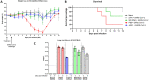
Update of
-
Prophylactic protection against respiratory viruses conferred by a prototype live attenuated influenza virus vaccine.bioRxiv [Preprint]. 2021 Apr 28:2021.04.28.441797. doi: 10.1101/2021.04.28.441797. bioRxiv. 2021. Update in: Sci Rep. 2021 Nov 12;11(1):22164. doi: 10.1038/s41598-021-01780-8. PMID: 33948589 Free PMC article. Updated. Preprint.
-
Prophylactic Protection Against Respiratory Viruses Conferred by a Prototype Live Attenuated Influenza Virus Vaccine.Res Sq [Preprint]. 2021 Aug 13:rs.3.rs-668116. doi: 10.21203/rs.3.rs-668116/v1. Res Sq. 2021. Update in: Sci Rep. 2021 Nov 12;11(1):22164. doi: 10.1038/s41598-021-01780-8. PMID: 34401874 Free PMC article. Updated. Preprint.
Similar articles
-
Prophylactic Protection Against Respiratory Viruses Conferred by a Prototype Live Attenuated Influenza Virus Vaccine.Res Sq [Preprint]. 2021 Aug 13:rs.3.rs-668116. doi: 10.21203/rs.3.rs-668116/v1. Res Sq. 2021. Update in: Sci Rep. 2021 Nov 12;11(1):22164. doi: 10.1038/s41598-021-01780-8. PMID: 34401874 Free PMC article. Updated. Preprint.
-
Prophylactic protection against respiratory viruses conferred by a prototype live attenuated influenza virus vaccine.bioRxiv [Preprint]. 2021 Apr 28:2021.04.28.441797. doi: 10.1101/2021.04.28.441797. bioRxiv. 2021. Update in: Sci Rep. 2021 Nov 12;11(1):22164. doi: 10.1038/s41598-021-01780-8. PMID: 33948589 Free PMC article. Updated. Preprint.
-
Generation of DelNS1 Influenza Viruses: a Strategy for Optimizing Live Attenuated Influenza Vaccines.mBio. 2019 Sep 17;10(5):e02180-19. doi: 10.1128/mBio.02180-19. mBio. 2019. PMID: 31530680 Free PMC article.
-
Attenuated influenza virus vaccines with modified NS1 proteins.Curr Top Microbiol Immunol. 2009;333:177-95. doi: 10.1007/978-3-540-92165-3_9. Curr Top Microbiol Immunol. 2009. PMID: 19768406 Review.
-
[Using reverse genetics method for developing recombinant strains of influenza viruses acceptable for use as live attenuated vaccines].Zh Mikrobiol Epidemiol Immunobiol. 2009 Mar-Apr;(2):111-7. Zh Mikrobiol Epidemiol Immunobiol. 2009. PMID: 19462520 Review. Russian.
Cited by
-
Fighting flu: novel CD8+ T-cell targets are required for future influenza vaccines.Clin Transl Immunology. 2024 Feb 14;13(2):e1491. doi: 10.1002/cti2.1491. eCollection 2024. Clin Transl Immunology. 2024. PMID: 38362528 Free PMC article. Review.
-
A defective viral genome strategy elicits broad protective immunity against respiratory viruses.Cell. 2021 Dec 9;184(25):6037-6051.e14. doi: 10.1016/j.cell.2021.11.023. Epub 2021 Nov 18. Cell. 2021. PMID: 34852237 Free PMC article.
-
Mucosal Immunization with an Influenza Vector Carrying SARS-CoV-2 N Protein Protects Naïve Mice and Prevents Disease Enhancement in Seropositive Th2-Prone Mice.Vaccines (Basel). 2024 Dec 28;13(1):15. doi: 10.3390/vaccines13010015. Vaccines (Basel). 2024. PMID: 39852794 Free PMC article.
-
Associated virus-bacterial vaccine based on seasonal LAIV and S. pneumoniae chimeric peptide provide protection against post-influenza pneumococcal infection in mouse model.Virulence. 2022 Dec;13(1):558-568. doi: 10.1080/21505594.2022.2049496. Virulence. 2022. PMID: 35266442 Free PMC article.
-
Defective Interfering Particles of Influenza Virus and Their Characteristics, Impacts, and Use in Vaccines and Antiviral Strategies: A Systematic Review.Viruses. 2022 Dec 12;14(12):2773. doi: 10.3390/v14122773. Viruses. 2022. PMID: 36560777 Free PMC article.
References
-
- Taft J, Bogunovic D. The Goldilocks zone of Type I IFNs: Lessons from human genetics. J. Immunol. 2018;201:3479–3485. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

