Impact of ceftolozane/tazobactam concentrations in continuous infusion against extensively drug-resistant Pseudomonas aeruginosa isolates in a hollow-fiber infection model
- PMID: 34773066
- PMCID: PMC8589991
- DOI: 10.1038/s41598-021-01784-4
Impact of ceftolozane/tazobactam concentrations in continuous infusion against extensively drug-resistant Pseudomonas aeruginosa isolates in a hollow-fiber infection model
Abstract
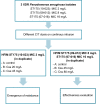
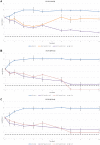
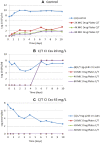
Ceftolozane/tazobactam (C/T) has emerged as a potential agent for the treatment of extensively drug-resistant (XDR) Pseudomonas aeruginosa infections. As it is a time-dependent antimicrobial, prolonged infusion may help achieve pharmacokinetic/pharmacodynamic (PK/PD) targets. To compare alternative steady-state concentrations (Css) of C/T in continuous infusion (CI) against three XDR P. aeruginosa ST175 isolates with C/T minimum inhibitory concentration (MIC) values of 2 to 16 mg/L in a hollow-fiber infection model (HFIM). Duplicate 10-day HFIM assays were performed to evaluate Css of C/T in CI: one compared 20 and 45 mg/L against the C/T-susceptible isolate while the other compared 45 and 80 mg/L against the two C/T-non-susceptible isolates. C/T resistance emerged when C/T-susceptible isolate was treated with C/T in CI at a Css of 20 mg/L; which showed a deletion in the gene encoding AmpC β-lactamase. The higher dosing regimen (80 mg/L) showed a slight advantage in effectiveness. The higher dosing regimen has the greatest bactericidal effect, regardless of C/T MIC. Exposure to the suboptimal Css of 20 mg/L led to the emergence of C/T resistance in the susceptible isolate. Antimicrobial regimens should be optimized through C/T levels monitoring and dose adjustments to improve clinical management.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Díaz-Cañestro M, Periañez L, Mulet X, Martin-Pena ML, Fraile-Ribot PA, Ayestarán I, et al. Ceftolozane/tazobactam for the treatment of multidrug resistant Pseudomonas aeruginosa: experience from the Balearic Islands. Eur. J. Clin. Microbiol. Infect. Dis. 2018;37:2191–2200. doi: 10.1007/s10096-018-3361-0. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

