Impact of high energy oral nutritional supplements consumed in the late afternoon on appetite, energy intake and cardio-metabolic risk factors in females with lower BMI
- PMID: 34773094
- PMCID: PMC9187517
- DOI: 10.1038/s41430-021-01042-w
Impact of high energy oral nutritional supplements consumed in the late afternoon on appetite, energy intake and cardio-metabolic risk factors in females with lower BMI
Abstract
Background/objective: Morning consumption of a single dose of high-energy oral nutritional supplement (ONS) in females with a lower BMI displaces some of the food eaten at breakfast but increases overall daily energy intake. This study investigated the effectiveness of ONS intake in the late afternoon and for longer duration.
Subjects/methods: Twenty-one healthy females (mean ± SD, age 25 ± 5 years; BMI 18.7 ± 1.2 kg/m2) participated in a randomised, crossover study with two experimental trials. In the afternoon of days 1-5, participants consumed either ONS (2.510 MJ) or low-energy PLACEBO drink (0.377 MJ) and recorded food eaten at home. On day six, energy intake was measured during buffet meals, and energy expenditure, appetite measurements and blood samples were collected throughout the day.
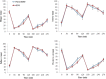
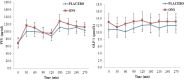
Result: Over the 5-day period, in the ONS trial energy intake from evening meals was lower (ONS, 2.7 ± 0.25 MJ; Placebo, 3.6 ± 0.25 MJ, P = 0.01) but averaged total daily energy intake was higher (ONS, 9.2 ± 0.3 MJ; PLACEBO, 8.2 ± 0.4 MJ, P = 0.03). On day six, energy intake, appetite scores, plasma GLP-1 and PYY, and energy expenditure were not significantly different between the two trials but fasting insulin concentration and HOMAIR, were higher (P < 0.05) and insulin sensitivity score based on fasting insulin and TAG lower (P < 0.05) in ONS trial.
Conclusion: Late afternoon consumption of ONS for five consecutive days by females with a lower BMI has only a partial and short-lived energy intake suppression and thus increases daily energy intake but reduces insulin sensitivity.
© 2021. Crown.
Conflict of interest statement
Outside the submitted work, KG has received research grants, expenses for conference attendance, consultancy and speaker fees paid by Nestle, Nutricia, and Dr Falk. None of the other authors has any relevant personal or financial conflict of interest to disclose.
Figures

References
-
- Avelino-Silva TJ, Jaluul O. Malnutrition in hospitalized older patients: management strategies to improve patient care and clinical outcomes. Int J Gerontol. 2017;11:56–61.
-
- Crichton M, Craven D, Mackay H, Marx W, de van der Schueren M, Marshall SA. Systematic review, meta-analysis and meta-regression of the prevalence of protein-energy malnutrition: Associations with geographical region and sex. Age Ageing. 2018;48:38–48. - PubMed
-
- Stratton RJ, Hebuterne X, Elia M. A systematic review and meta-analysis of the impact of oral nutritional supplements on hospital readmissions. Ageing Res Rev. 2013;12:884–97. - PubMed
-
- Bally MR, Yildirim PZB, Bounoure L, Gloy VL, Mueller B, Briel M, et al. Nutritional support and outcomes in malnourished medical inpatients: a systematic review and meta-analysis. JAMA Intern Med. 2016;176:43–53. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

