Melanoma secretion of transforming growth factor-β2 leads to loss of epidermal AMBRA1 threatening epidermal integrity and facilitating tumour ulceration
- PMID: 34773645
- PMCID: PMC9546516
- DOI: 10.1111/bjd.20889
Melanoma secretion of transforming growth factor-β2 leads to loss of epidermal AMBRA1 threatening epidermal integrity and facilitating tumour ulceration
Abstract
Background: For patients with early American Joint Committee on Cancer (AJCC)-stage melanoma the combined loss of the autophagy regulatory protein AMBRA1 and the terminal differentiation marker loricrin in the peritumoral epidermis is associated with a significantly increased risk of metastasis.
Objectives: The aim of the present study was to evaluate the potential contribution of melanoma paracrine transforming growth factor (TGF)-β signalling to the loss of AMBRA1 in the epidermis overlying the primary tumour and disruption of epidermal integrity.
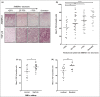
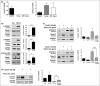
Methods: Immunohistochemistry was used to analyse AMBRA1 and TGF-β2 in a cohort of 109 AJCC all-stage melanomas, and TGF-β2 and claudin-1 in a cohort of 30 or 42 AJCC stage I melanomas, respectively, with known AMBRA1 and loricrin (AMLo) expression. Evidence of pre-ulceration was analysed in a cohort of 42 melanomas, with TGF-β2 signalling evaluated in primary keratinocytes.
Results: Increased tumoral TGF-β2 was significantly associated with loss of peritumoral AMBRA1 (P < 0·05), ulceration (P < 0·001), AMLo high-risk status (P < 0·05) and metastasis (P < 0·01). TGF-β2 treatment of keratinocytes resulted in downregulation of AMBRA1, loricrin and claudin-1, while knockdown of AMBRA1 was associated with decreased expression of claudin-1 and increased proliferation of keratinocytes (P < 0·05). Importantly, we show loss of AMBRA1 in the peritumoral epidermis was associated with decreased claudin-1 expression (P < 0·05), parakeratosis (P < 0·01) and cleft formation in the dermoepidermal junction (P < 0·05).
Conclusions: Collectively, these data suggest a paracrine mechanism whereby TGF-β2 causes loss of AMBRA1 overlying high-risk AJCC early-stage melanomas and reduced epidermal integrity, thereby facilitating erosion of the epidermis and tumour ulceration.
© 2021 The Authors. British Journal of Dermatology published by John Wiley & Sons Ltd on behalf of British Association of Dermatologists.
Figures




Comment in
-
Epidermal melanoma prognostic factors: a link to paracrine transforming growth factor-β signalling.Br J Dermatol. 2022 Apr;186(4):606-607. doi: 10.1111/bjd.20981. Epub 2022 Mar 2. Br J Dermatol. 2022. PMID: 35233773 No abstract available.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

