In vivo dynamics of acidosis and oxidative stress in the acute phase of an ischemic stroke in a rodent model
- PMID: 34773835
- PMCID: PMC8600061
- DOI: 10.1016/j.redox.2021.102178
In vivo dynamics of acidosis and oxidative stress in the acute phase of an ischemic stroke in a rodent model
Abstract
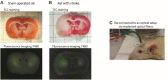
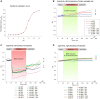
Ischemic cerebral stroke is one of the leading causes of death and disability in humans. However, molecular processes underlying the development of this pathology remain poorly understood. There are major gaps in our understanding of metabolic changes that occur in the brain tissue during the early stages of ischemia and reperfusion. In particular, it is generally accepted that both ischemia (I) and reperfusion (R) generate reactive oxygen species (ROS) that cause oxidative stress which is one of the main drivers of the pathology, although ROS generation during I/R was never demonstrated in vivo due to the lack of suitable methods. In the present study, we record for the first time the dynamics of intracellular pH and H2O2 during I/R in cultured neurons and during experimental stroke in rats using the latest generation of genetically encoded biosensors SypHer3s and HyPer7. We detect a buildup of powerful acidosis in the brain tissue that overlaps with the ischemic core from the first seconds of pathogenesis. At the same time, no significant H2O2 generation was found in the acute phase of ischemia/reperfusion. HyPer7 oxidation in the brain was detected only 24 h later. Comparison of in vivo experiments with studies on cultured neurons under I/R demonstrates that the dynamics of metabolic processes in these models significantly differ, suggesting that a cell culture is a poor predictor of metabolic events in vivo.
Keywords: Genetically encoded fluorescent biosensors; Hydrogen peroxide; Hypoxia/reoxygenation; In vivo optical brain interrogation; Ischemia/reperfusion; Ischemic stroke.
Copyright © 2021 The Authors. Published by Elsevier B.V. All rights reserved.
Figures
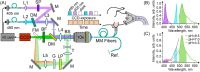


References
LinkOut - more resources
Full Text Sources
Research Materials

