Meropenem pharmacokinetics in critically ill patients with or without burn treated with or without continuous veno-venous haemofiltration
- PMID: 34773921
- PMCID: PMC9299819
- DOI: 10.1111/bcp.15138
Meropenem pharmacokinetics in critically ill patients with or without burn treated with or without continuous veno-venous haemofiltration
Abstract
Introduction: Severe burn injury involves widespread skin and tissue damage leading to systemic inflammation, hypermetabolism and multi-organ failure. The hypermetabolic phase of burn injury has been associated with increased systemic antibiotic clearance; however, critical illness in the absence of burn may also induce similar physiologic changes. Continuous renal replacement therapy (CRRT) is often implemented in critically ill patients and may also affect antibiotic clearance. Although the pharmacokinetics (PK) of meropenem has been described in both the burn and non-burn critically ill populations, direct comparative data is lacking.
Methods: For this study, we evaluated PK parameters of meropenem from 23 critically ill patients, burn or non-burn, treated with or without continuous veno-venous haemofiltration (CVVH) to determine the contribution of burn and CVVH to the variability of therapeutic meropenem levels.
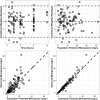
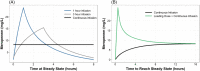
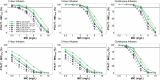
Results: A two-compartment model best described the data and revealed creatinine clearance (CrCl) and total burn surface area (TBSA) as significant covariates on clearance (CL) and peripheral volume of distribution (Vp), respectively. Of interest, non-burn patients on CVVH displayed an overall lower inherent CL as compared to burn patients on CVVH (6.43 vs. 12.85 L/h). Probability of target attainment (PTA) simulations revealed augmented renal clearance (ARC) may necessitate dose adjustments, but TBSA and CVVH would not.
Conclusions: We recommend a standard dose of 1000 mg every 8 hours; however, if ARC is suspected, or the severity of illness requires a more stringent therapeutic target, we recommend a loading dose of 1000-2000 mg infused over 30 minutes to 1 hour followed by continuous infusion (3000-6000 mg over 24 hours), or intermittent infusion of 2000 mg every 8 hours.
Keywords: burns; continuous renal replacement therapy; critical illness; meropenem; pharmacokinetics.
Published 2021. This article is a U.S. Government work and is in the public domain in the USA. British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
The authors have no conflicts of interest to report. Material has been reviewed by the Walter Reed Army Institute of Research, the Uniformed Services University of the Health Sciences and the United States Institute of Surgical Research. There is no objection to its presentation and/or publication. The views expressed in this article are those of the authors and do not reflect the official policy or position of the US Army Medical Department, Department of the Army, DoD or the US Government.
Figures

References
-
- Merchant M, Kim C, Grossman P. Acute Kidney Injury and CRRT in Burn Patients. Proceedings of UCLA Health; 2018:22.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

