The relationship between blood-based tumor mutation burden level and efficacy of PD-1/PD-L1 inhibitors in advanced non-small cell lung cancer: a systematic review and meta-analysis
- PMID: 34774004
- PMCID: PMC8590772
- DOI: 10.1186/s12885-021-08924-z
The relationship between blood-based tumor mutation burden level and efficacy of PD-1/PD-L1 inhibitors in advanced non-small cell lung cancer: a systematic review and meta-analysis
Abstract
Background: The predictive role of blood-based tumor mutation burden (bTMB) for selecting advanced nonsmall cell lung cancer (NSCLC) patients who might benefit from immune checkpoint inhibitors (ICIs) is still under debate. Therefore, the purpose of this meta-analysis was to evaluate the efficacy of programmed cell death 1 (PD-1) /programmed cell death ligand 1 (PD-L1) inhibitors versus that of standard-of-care therapy in patients with NSCLC who were bTMB high and bTMB low.
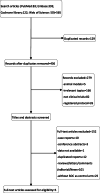
Methods: PubMed, Embase, Cochrane, the Web of Science, and ClinicalTrials.gov were searched systematically from inception to February 2021 for studies of PD-1/PD-L1 inhibitors (durvalumab OR atezolizumab OR avelumab OR pembrolizumab OR Nivolumab) that provided hazard ratios (HRs) for overall survival (OS) or progression-free survival (PFS), or odds ratios (ORs) for objective response rate (ORR) in both bTMB high and bTMB low groups.
Results: A total of 2338 patients with advanced or metastatic NSCLC from six randomized controlled trials, which all used chemotherapy (CT) as a control, were included in this study. Compared with CT, PD-1/PD-L1 inhibitor therapy improved OS (HR 0.62, 95% CI 0.52-0.75, P < 0.01), PFS (HR 0.57, 95% CI 0.48-0.67, P < 0.01), and ORR (OR 2.69, 95% CI 1.84-3.93, P < 0.01) in bTMB-high NSCLC patients but not in bTMB-low patients (OS HR 0.86, 95% CI 0.69-1.07, P = 0.17; PFS HR 1.00, 95% CI 0.78-1.27, P = 0.98; ORR OR 0.63, 95% CI 0.49-0.80, P = 0.03). Subgroup analyses showed that these results were consistent across all subgroups (line of therapy, therapy regimen, type of NGS panel, PD-L1 expression, and cutoff value). Meta-regression analysis showed that the proportion of patients with squamous cell histology had no statistical effect on clinical outcomes. Sensitivity analyses illustrated that all results were stable.
Conclusions: The efficacy of PD-1/PD-L1 inhibitor therapy in advanced NSCLC patients may be dependent on bTMB level. Patients with high bTMB tend to obtain significantly better OS, PFS, and ORR from PD-1/PD-L1 inhibitor therapy than from CT. However, because of multiple limitations, including those related to reproducibility, the results are exploratory and should be interpreted with caution.
Keywords: Blood; Lung cancer; Meta-analysis; NSCLC; PD-1; PD-L1; Survival; Tumor mutation burden.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Single or combined immune checkpoint inhibitors compared to first-line platinum-based chemotherapy with or without bevacizumab for people with advanced non-small cell lung cancer.Cochrane Database Syst Rev. 2020 Dec 14;12(12):CD013257. doi: 10.1002/14651858.CD013257.pub2. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 30;4:CD013257. doi: 10.1002/14651858.CD013257.pub3. PMID: 33316104 Free PMC article. Updated.
-
Single or combined immune checkpoint inhibitors compared to first-line platinum-based chemotherapy with or without bevacizumab for people with advanced non-small cell lung cancer.Cochrane Database Syst Rev. 2021 Apr 30;4(4):CD013257. doi: 10.1002/14651858.CD013257.pub3. Cochrane Database Syst Rev. 2021. PMID: 33930176 Free PMC article.
-
Association of Survival and Immune-Related Biomarkers With Immunotherapy in Patients With Non-Small Cell Lung Cancer: A Meta-analysis and Individual Patient-Level Analysis.JAMA Netw Open. 2019 Jul 3;2(7):e196879. doi: 10.1001/jamanetworkopen.2019.6879. JAMA Netw Open. 2019. PMID: 31290993 Free PMC article.
-
Comparative analysis of immune checkpoint inhibitors and chemotherapy in the treatment of advanced non-small cell lung cancer: A meta-analysis of randomized controlled trials.Medicine (Baltimore). 2018 Aug;97(33):e11936. doi: 10.1097/MD.0000000000011936. Medicine (Baltimore). 2018. PMID: 30113497 Free PMC article. Review.
-
A meta-analysis of efficacy and safety of antibodies targeting PD-1/PD-L1 in treatment of advanced nonsmall cell lung cancer.Medicine (Baltimore). 2016 Dec;95(52):e5539. doi: 10.1097/MD.0000000000005539. Medicine (Baltimore). 2016. PMID: 28033249 Free PMC article. Review.
Cited by
-
Comparison of efficacy and tolerability of adjuvant therapy for resected high-risk stage III-IV cutaneous melanoma: a systemic review and Bayesian network meta-analysis.Ther Adv Med Oncol. 2023 Jan 24;15:17588359221148918. doi: 10.1177/17588359221148918. eCollection 2023. Ther Adv Med Oncol. 2023. PMID: 36743526 Free PMC article.
-
Cuproptosis-related lncRNA scoring system to predict the clinical outcome and immune landscape in pancreatic adenocarcinoma.Sci Rep. 2023 Nov 27;13(1):20870. doi: 10.1038/s41598-023-47223-4. Sci Rep. 2023. PMID: 38012210 Free PMC article.
-
Atezolizumab Plus Bevacizumab as First-line Treatment for Patients With Metastatic Nonsquamous Non-Small Cell Lung Cancer With High Tumor Mutation Burden: A Nonrandomized Controlled Trial.JAMA Oncol. 2023 Mar 1;9(3):344-353. doi: 10.1001/jamaoncol.2022.5959. JAMA Oncol. 2023. PMID: 36520426 Free PMC article.
-
Circulating Tumor DNA-Based Genomic Profiling Assays in Adult Solid Tumors for Precision Oncology: Recent Advancements and Future Challenges.Cancers (Basel). 2022 Jul 4;14(13):3275. doi: 10.3390/cancers14133275. Cancers (Basel). 2022. PMID: 35805046 Free PMC article. Review.
-
Optimal Therapeutic Strategy for PD-L1 Negative Metastatic Non-Small Cell Lung Cancer: A Decision-Making Guide Based on Clinicopathological and Molecular Features.Curr Treat Options Oncol. 2023 Nov;24(11):1550-1567. doi: 10.1007/s11864-023-01132-w. Epub 2023 Oct 6. Curr Treat Options Oncol. 2023. PMID: 37801207 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

