Tumor-immune profiling of CT-26 and Colon 26 syngeneic mouse models reveals mechanism of anti-PD-1 response
- PMID: 34774008
- PMCID: PMC8590766
- DOI: 10.1186/s12885-021-08974-3
Tumor-immune profiling of CT-26 and Colon 26 syngeneic mouse models reveals mechanism of anti-PD-1 response
Abstract
Background: Immune checkpoint blockade (ICB) therapies have changed the paradigm of cancer therapies. However, anti-tumor response of the ICB is insufficient for many patients and limited to specific tumor types. Despite many preclinical and clinical studies to understand the mechanism of anti-tumor efficacy of ICB, the mechanism is not completely understood. Harnessing preclinical tumor models is one way to understand the mechanism of treatment response.
Methods: In order to delineate the mechanisms of anti-tumor activity of ICB in preclinical syngeneic tumor models, we selected two syngeneic murine colorectal cancer models based on in vivo screening for sensitivity with anti-PD-1 therapy. We performed tumor-immune profiling of the two models to identify the potential mechanism for anti-PD-1 response.
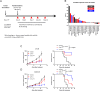
Results: We performed in vivo screening for anti-PD-1 therapy across 23 syngeneic tumor models and found that CT-26 and Colon 26, which are murine colorectal carcinoma derived from BALB/c mice, showed different sensitivity to anti-PD-1. CT-26 tumor mice were more sensitive to the anti-PD-1 antibody than Colon 26, while both models show similarly sensitivity to anti-CTLA4 antibody. Immune-profiling showed that CT-26 tumor tissue was infiltrated with more immune cells than Colon 26. Genomic/transcriptomic analyses highlighted thatWnt pathway was one of the potential differences between CT-26 and Colon 26, showing Wnt activity was higher in Colon 26 than CT-26. .
Conclusions: CT-26 and Colon 26 syngeneic tumor models showed different sensitivity to anti-PD-1 therapy, although both tumor cells are murine colorectal carcinoma cell lines from BALB/c strain. By characterizing the mouse cells lines and tumor-immune context in the tumor tissues with comprehensive analysis approaches, we found that CT-26 showed "hot tumor" profile with more infiltrated immune cells than Colon 26. Further pathway analyses enable us to propose a hypothesis that Wnt pathway could be one of the major factors to differentiate CT-26 from Colon 26 model and link to anti-PD-1 response. Our approach to focus on preclinical tumor models with similar genetic background but different sensitivity to anti-PD-1 therapy would contribute to illustrating the potential mechanism of anti-PD-1 response and to generating a novel concept to synergize current anti-PD-1 therapies for cancer patients.
Keywords: Anti-PD-1; Anti-tumor activity; CT-26; Colon 26; Immune checkpoint blockade; Syngeneic model; Wnt.
© 2021. The Author(s).
Conflict of interest statement
YS, MYL and MS are all employees of Millennium Pharmaceuticals, Inc. a wholly owned subsidiary of Takeda Pharmaceutical Company Limited, Cambridge, MA, USA. YF and HL were employees of Takeda when the work was being conducted.
Figures




References
-
- Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8(9):1069–1086. doi: 10.1158/2159-8290.CD-18-0367. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

