Assessment of retinal oxygen metabolism, visual function, thickness and degeneration markers after variable ischemia/reperfusion in rats
- PMID: 34774489
- PMCID: PMC8665131
- DOI: 10.1016/j.exer.2021.108838
Assessment of retinal oxygen metabolism, visual function, thickness and degeneration markers after variable ischemia/reperfusion in rats
Abstract
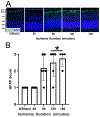
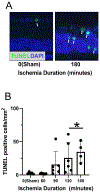
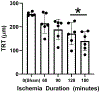
After total retinal ischemia induced experimentally by ophthalmic vessel occlusion followed by reperfusion, studies have reported alterations in retinal oxygen metabolism (MO2), delivery (DO2), and extraction fraction (OEF), as well as visual dysfunction and cell loss. In the current study, under variable durations of ischemia/reperfusion, changes in these oxygen metrics, visual function, retinal thickness, and degeneration markers (gliosis and apoptosis) were assessed and related. Additionally, the prognostic value of MO2 for predicting visual function and retinal thickness outcomes was reported. Sixty-one rats were divided into 5 groups of ischemia duration (0 [sham], 60, 90, 120, or 180 min) and 2 reperfusion durations (1 h, 7 days). Phosphorescence lifetime and blood flow imaging, electroretinography, and optical coherence tomography were performed. MO2 reduction was related to visual dysfunction, retinal thinning, increased gliosis and apoptosis after 7-days reperfusion. Impairment in MO2 after 1-h reperfusion predicted visual function and retinal thickness outcomes after 7-days reperfusion. Since MO2 can be measured in humans, findings from analogous studies may find value in the clinical setting.
Keywords: Ophthalmic vessel occlusion; Retinal ischemia; Retinal oxygen metabolism.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Disclosure
MS holds a patent for the oxygen imaging technology. The authors have no conflict of interest.
Figures

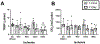
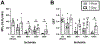
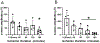

References
-
- Behzadian MA, Wang XL, Windsor LJ, Ghaly N, Caldwell RB, 2001. TGF-beta increases retinal endothelial cell permeability by increasing MMP-9: possible role of glial cells in endothelial barrier function. Invest Ophthalmol Vis Sci 42, 853–859. - PubMed
-
- Bhisitkul RB, Robinson GS, Moulton RS, Claffey KP, Gragoudas ES, Miller JW, 2005. An antisense oligodeoxynucleotide against vascular endothelial growth factor in a nonhuman primate model of iris neovascularization. Arch Ophthalmol 123, 214–219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

