Computational modeling predicts ephemeral acidic microdomains in the glutamatergic synaptic cleft
- PMID: 34774503
- PMCID: PMC8715252
- DOI: 10.1016/j.bpj.2021.11.011
Computational modeling predicts ephemeral acidic microdomains in the glutamatergic synaptic cleft
Abstract
At chemical synapses, synaptic vesicles release their acidic contents into the cleft, leading to the expectation that the cleft should acidify. However, fluorescent pH probes targeted to the cleft of conventional glutamatergic synapses in both fruit flies and mice reveal cleft alkalinization rather than acidification. Here, using a reaction-diffusion scheme, we modeled pH dynamics at the Drosophila neuromuscular junction as glutamate, ATP, and protons (H+) were released into the cleft. The model incorporates bicarbonate and phosphate buffering systems as well as plasma membrane calcium-ATPase activity and predicts substantial cleft acidification but only for fractions of a millisecond after neurotransmitter release. Thereafter, the cleft rapidly alkalinizes and remains alkaline for over 100 ms because the plasma membrane calcium-ATPase removes H+ from the cleft in exchange for calcium ions from adjacent pre- and postsynaptic compartments, thus recapitulating the empirical data. The extent of synaptic vesicle loading and time course of exocytosis have little influence on the magnitude of acidification. Phosphate but not bicarbonate buffering is effective at suppressing the magnitude and time course of the acid spike, whereas both buffering systems are effective at suppressing cleft alkalinization. The small volume of the cleft levies a powerful influence on the magnitude of alkalinization and its time course. Structural features that open the cleft to adjacent spaces appear to be essential for alleviating the extent of pH transients accompanying neurotransmission.
Copyright © 2021 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
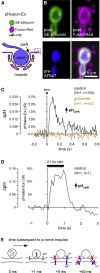



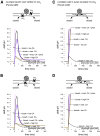
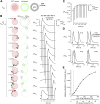
Comment in
-
To alkalinize or acidify, that is the question.Biophys J. 2021 Dec 21;120(24):5436-5437. doi: 10.1016/j.bpj.2021.11.012. Epub 2021 Nov 11. Biophys J. 2021. PMID: 34798062 Free PMC article. No abstract available.
Similar articles
-
Postsynaptic Calcium Extrusion at the Mouse Neuromuscular Junction Alkalinizes the Synaptic Cleft.J Neurosci. 2023 Aug 9;43(32):5741-5752. doi: 10.1523/JNEUROSCI.0815-23.2023. Epub 2023 Jul 20. J Neurosci. 2023. PMID: 37474311 Free PMC article.
-
Neuronal Glutamatergic Synaptic Clefts Alkalinize Rather Than Acidify during Neurotransmission.J Neurosci. 2020 Feb 19;40(8):1611-1624. doi: 10.1523/JNEUROSCI.1774-19.2020. Epub 2020 Jan 21. J Neurosci. 2020. PMID: 31964719 Free PMC article.
-
Differential control of synaptic and ectopic vesicular release of glutamate.J Neurosci. 2004 Oct 13;24(41):8932-9. doi: 10.1523/JNEUROSCI.2650-04.2004. J Neurosci. 2004. PMID: 15483112 Free PMC article.
-
The sequence of events that underlie quantal transmission at central glutamatergic synapses.Nat Rev Neurosci. 2007 Aug;8(8):597-609. doi: 10.1038/nrn2191. Nat Rev Neurosci. 2007. PMID: 17637801 Review.
-
Distinct Calcium Sources Define Compartmentalized Synaptic Signaling Domains.Neuroscientist. 2019 Oct;25(5):408-419. doi: 10.1177/1073858419863771. Epub 2019 Aug 2. Neuroscientist. 2019. PMID: 31375041 Review.
Cited by
-
Postsynaptic Calcium Extrusion at the Mouse Neuromuscular Junction Alkalinizes the Synaptic Cleft.J Neurosci. 2023 Aug 9;43(32):5741-5752. doi: 10.1523/JNEUROSCI.0815-23.2023. Epub 2023 Jul 20. J Neurosci. 2023. PMID: 37474311 Free PMC article.
-
Monomeric α-synuclein activates the plasma membrane calcium pump.EMBO J. 2023 Dec 1;42(23):e111122. doi: 10.15252/embj.2022111122. Epub 2023 Nov 2. EMBO J. 2023. PMID: 37916890 Free PMC article.
-
SLC4A10 mutation causes a neurological disorder associated with impaired GABAergic transmission.Brain. 2023 Nov 2;146(11):4547-4561. doi: 10.1093/brain/awad235. Brain. 2023. PMID: 37459438 Free PMC article.
-
Proton-triggered rearrangement of the AMPA receptor N-terminal domains impacts receptor kinetics and synaptic localization.Nat Struct Mol Biol. 2024 Oct;31(10):1601-1613. doi: 10.1038/s41594-024-01369-5. Epub 2024 Aug 13. Nat Struct Mol Biol. 2024. PMID: 39138332 Free PMC article.
References
-
- Chesler M. Regulation and modulation of pH in the brain. Physiol. Rev. 2003;83:1183–1221. - PubMed
-
- Obara M., Szeliga M., Albrecht J. Regulation of pH in the mammalian central nervous system under normal and pathological conditions: facts and hypotheses. Neurochem. Int. 2008;52:905–919. - PubMed
-
- Sinning A., Hübner C.A. Minireview: pH and synaptic transmission. FEBS Lett. 2013;587:1923–1928. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

