Randomized Controlled-Feeding Study of Dietary Emulsifier Carboxymethylcellulose Reveals Detrimental Impacts on the Gut Microbiota and Metabolome
- PMID: 34774538
- PMCID: PMC9639366
- DOI: 10.1053/j.gastro.2021.11.006
Randomized Controlled-Feeding Study of Dietary Emulsifier Carboxymethylcellulose Reveals Detrimental Impacts on the Gut Microbiota and Metabolome
Abstract
Background & aims: Epidemiologic and murine studies suggest that dietary emulsifiers promote development of diseases associated with microbiota dysbiosis. Although the detrimental impact of these compounds on the intestinal microbiota and intestinal health have been demonstrated in animal and in vitro models, impact of these food additives in healthy humans remains poorly characterized.
Methods: To examine this notion in humans, we performed a double-blind controlled-feeding study of the ubiquitous synthetic emulsifier carboxymethylcellulose (CMC) in which healthy adults consumed only emulsifier-free diets (n = 9) or an identical diet enriched with 15 g per day of CMC (n = 7) for 11 days.
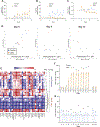
Results: Relative to control subjects, CMC consumption modestly increased postprandial abdominal discomfort and perturbed gut microbiota composition in a way that reduced its diversity. Moreover, CMC-fed subjects exhibited changes in the fecal metabolome, particularly reductions in short-chain fatty acids and free amino acids. Furthermore, we identified 2 subjects consuming CMC who exhibited increased microbiota encroachment into the normally sterile inner mucus layer, a central feature of gut inflammation, as well as stark alterations in microbiota composition.
Conclusions: These results support the notion that the broad use of CMC in processed foods may be contributing to increased prevalence of an array of chronic inflammatory diseases by altering the gut microbiome and metabolome (ClinicalTrials.gov, number NCT03440229).
Keywords: Emulsifier; Metabolism; Metabolome; Microbiota.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest
Dr Lewis consulted or served on an advisory board for Eli Lilly and Company, Samsung Bioepis, UCB, Bristol-Myers Squibb, Nestle Health Science, Merck, Celgene, Janssen Pharmaceuticals, Bridge Biotherapeutics, Entasis Therapeutics, AbbVie, Pfizer, Gilead, Arena Pharmaceuticals, Protagonist Therapeutics, Amgen, and Scipher Medicine. He has had research funding from Nestle Health Science, Takeda, Janssen Pharmaceuticals, and AbbVie. The remaining authors disclose no conflicts.
Figures




Comment in
-
The Role of Carboxymethylcellulose in Health and Disease: Is the Plot Thickening?Gastroenterology. 2022 Sep;163(3):780-781. doi: 10.1053/j.gastro.2022.01.007. Epub 2022 Jan 11. Gastroenterology. 2022. PMID: 35026236 No abstract available.
References
-
- Martinez Steele E, Juul F, Neri D, et al. Dietary share of ultra-processed foods and metabolic syndrome in the US adult population. Prev Med 2019;125:40–48. - PubMed
-
- Caruso R, Lo BC, Nunez G. Host-microbiota interactions in inflammatory bowel disease. Nat Rev Immunol 2020;20:411–426. - PubMed
-
- Neurath MF. Host-microbiota interactions in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol 2020;17:76–77. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

