Nanoparticular CpG-adjuvanted SARS-CoV-2 S1 protein elicits broadly neutralizing and Th1-biased immunoreactivity in mice
- PMID: 34774590
- PMCID: PMC8580573
- DOI: 10.1016/j.ijbiomac.2021.11.020
Nanoparticular CpG-adjuvanted SARS-CoV-2 S1 protein elicits broadly neutralizing and Th1-biased immunoreactivity in mice
Abstract
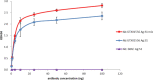
The spike (S) protein is a leading vaccine candidate against SARS-CoV-2 infection. The S1 domain of S protein, which contains a critical receptor-binding domain (RBD) antigen, potentially induces protective immunoreactivities against SARS-CoV-2. In this study, we presented preclinical evaluations of a novel insect cell-derived SARS-CoV-2 recombinant S1 (rS1) protein as a potent COVID-19 vaccine candidate. The native antigenicity of rS1 was characterized by enzyme-linked immunosorbent assay with a neutralizing monoclonal antibody targeting the RBD antigen. To improve its immunogenicity, rS1-adjuvanted with fucoidan/trimethylchitosan nanoparticles (FUC-TMC NPs) and cytosine-phosphate-guanosine-oligodeoxynucleotides (CpG-ODNs) were investigated using a mouse model. The S1-specific immunoglobulin G (IgG) titers, FluoroSpot assay, pseudovirus- and prototype SARS-CoV-2-based neutralization assays were assessed. The results showed that the rS1/CpG/ FUC-TMC NPs (rS1/CpG/NPs) formulation induced a broad-spectrum IgG response with potent, long-lasting, and cross-protective neutralizing activity against the emerging SARS-CoV-2 variant of concern, along with a Th1-biased cellular response. Thus, the rS1/CpG/NPs formulation presents a promising vaccination approach against COVID-19.
Keywords: CpG adjuvant; Fucoidan-trimethylchitosan nanoparticles; Immunogenicity enhancement; Neutralizing antibody; Recombinant S1 protein; SARS-CoV-2 infection; Th1-biased cellular immune responses.
Copyright © 2021. Published by Elsevier B.V.
Figures


Similar articles
-
Elicitation of Broadly Neutralizing Antibodies against B.1.1.7, B.1.351, and B.1.617.1 SARS-CoV-2 Variants by Three Prototype Strain-Derived Recombinant Protein Vaccines.Viruses. 2021 Jul 22;13(8):1421. doi: 10.3390/v13081421. Viruses. 2021. PMID: 34452287 Free PMC article.
-
Self-assembled epitope-based nanoparticles targeting the SARS-CoV-2 spike protein enhanced the immune response and induced potential broad neutralizing activity.Front Cell Infect Microbiol. 2025 Apr 9;15:1560330. doi: 10.3389/fcimb.2025.1560330. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40270771 Free PMC article.
-
SARS-CoV-2 S1 is superior to the RBD as a COVID-19 subunit vaccine antigen.J Med Virol. 2021 Feb;93(2):892-898. doi: 10.1002/jmv.26320. Epub 2020 Oct 5. J Med Virol. 2021. PMID: 32691875 Free PMC article.
-
COVID-19 antibody development fueled by HIV-1 broadly neutralizing antibody research.Curr Opin HIV AIDS. 2021 Jan;16(1):25-35. doi: 10.1097/COH.0000000000000657. Curr Opin HIV AIDS. 2021. PMID: 33229949 Free PMC article. Review.
-
The recent advances in vaccine adjuvants.Front Immunol. 2025 May 13;16:1557415. doi: 10.3389/fimmu.2025.1557415. eCollection 2025. Front Immunol. 2025. PMID: 40433383 Free PMC article. Review.
Cited by
-
Immunopotentiating Activity of Fucoidans and Relevance to Cancer Immunotherapy.Mar Drugs. 2023 Feb 15;21(2):128. doi: 10.3390/md21020128. Mar Drugs. 2023. PMID: 36827169 Free PMC article. Review.
-
Computer-Based Immunoinformatic Analysis to Predict Candidate T-Cell Epitopes for SARS-CoV-2 Vaccine Design.Front Immunol. 2022 Mar 30;13:847617. doi: 10.3389/fimmu.2022.847617. eCollection 2022. Front Immunol. 2022. PMID: 35432316 Free PMC article.
-
A state-of-the-art review on fucoidan as an antiviral agent to combat viral infections.Carbohydr Polym. 2022 Sep 1;291:119551. doi: 10.1016/j.carbpol.2022.119551. Epub 2022 May 2. Carbohydr Polym. 2022. PMID: 35698330 Free PMC article. Review.
-
A Synthetic SARS-CoV-2-Derived T-Cell and B-Cell Peptide Cocktail Elicits Full Protection against Lethal Omicron BA.1 Infection in H11-K18-hACE2 Mice.Microbiol Spectr. 2023 Mar 13;11(2):e0419422. doi: 10.1128/spectrum.04194-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36912685 Free PMC article.
-
Application of Baculovirus Expression Vector system (BEV) for COVID-19 diagnostics and therapeutics: a review.J Genet Eng Biotechnol. 2022 Jul 6;20(1):98. doi: 10.1186/s43141-022-00368-7. J Genet Eng Biotechnol. 2022. PMID: 35792966 Free PMC article. Review.
References
-
- Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Marc G.P., Moreira E.D., Zerbini C., Bailey R., Swanson K.A., Roychoudhury S., Koury K., Li P., Kalina W.V., Cooper D., Frenck R.W., Jr., Hammitt L.L., Türeci Ö., Nell H., Schaefer A., Ünal S., Tresnan D.B., Mather S., Dormitzer P.R., Şahin U., Jansen K.U., Gruber W.C. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. - DOI - PMC - PubMed
-
- Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., Bibi S., Briner C., Cicconi P., Collins A.M., Colin-Jones R., Cutland C.L., Darton T.C., Dheda K., Duncan C.J.A., Emary K.R.W., Ewer K.J., Fairlie L., Faust S.N., Feng S., Ferreira D.M., Finn A., Goodman A.L., Green C.M., Green C.A., Heath P.T., Hill C., Hill H., Hirsch I., Hodgson S.H.C., Izu A., Jackson S., Jenkin D., Joe C.C.D., Kerridge S., Koen A., Kwatra G., Lazarus R., Lawrie A.M., Lelliott A., Libri V., Lillie P.J., Mallory R., Mendes A.V.A., Milan E.P., Minassian A.M., McGregor A., Morrison H., Mujadidi Y.F., Nana A., O’Reilly P.J., Padayachee S.D., Pittella A., Plested E., Pollock K.M., Ramasamy M.N., Rhead S., Schwarzbold A.V., Singh N., Smith A., Song R., Snape M.D., Sprinz E., Sutherland R.K., Tarrant R., Thomson E.C., Török M.E., Toshner M., Turner D.P.J., Villafana T.L., Watson M.E.E., Williams C.J., Douglas A.D., Hill A.V.S., Lambe T., Gilbert S.C., Pollard A.J. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

