Nanoparticular CpG-adjuvanted SARS-CoV-2 S1 protein elicits broadly neutralizing and Th1-biased immunoreactivity in mice
- PMID: 34774590
- PMCID: PMC8580573
- DOI: 10.1016/j.ijbiomac.2021.11.020
Nanoparticular CpG-adjuvanted SARS-CoV-2 S1 protein elicits broadly neutralizing and Th1-biased immunoreactivity in mice
Abstract
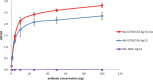
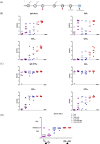
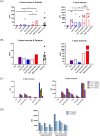
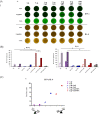
The spike (S) protein is a leading vaccine candidate against SARS-CoV-2 infection. The S1 domain of S protein, which contains a critical receptor-binding domain (RBD) antigen, potentially induces protective immunoreactivities against SARS-CoV-2. In this study, we presented preclinical evaluations of a novel insect cell-derived SARS-CoV-2 recombinant S1 (rS1) protein as a potent COVID-19 vaccine candidate. The native antigenicity of rS1 was characterized by enzyme-linked immunosorbent assay with a neutralizing monoclonal antibody targeting the RBD antigen. To improve its immunogenicity, rS1-adjuvanted with fucoidan/trimethylchitosan nanoparticles (FUC-TMC NPs) and cytosine-phosphate-guanosine-oligodeoxynucleotides (CpG-ODNs) were investigated using a mouse model. The S1-specific immunoglobulin G (IgG) titers, FluoroSpot assay, pseudovirus- and prototype SARS-CoV-2-based neutralization assays were assessed. The results showed that the rS1/CpG/ FUC-TMC NPs (rS1/CpG/NPs) formulation induced a broad-spectrum IgG response with potent, long-lasting, and cross-protective neutralizing activity against the emerging SARS-CoV-2 variant of concern, along with a Th1-biased cellular response. Thus, the rS1/CpG/NPs formulation presents a promising vaccination approach against COVID-19.
Keywords: CpG adjuvant; Fucoidan-trimethylchitosan nanoparticles; Immunogenicity enhancement; Neutralizing antibody; Recombinant S1 protein; SARS-CoV-2 infection; Th1-biased cellular immune responses.
Copyright © 2021. Published by Elsevier B.V.
Figures


References
-
- Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Marc G.P., Moreira E.D., Zerbini C., Bailey R., Swanson K.A., Roychoudhury S., Koury K., Li P., Kalina W.V., Cooper D., Frenck R.W., Jr., Hammitt L.L., Türeci Ö., Nell H., Schaefer A., Ünal S., Tresnan D.B., Mather S., Dormitzer P.R., Şahin U., Jansen K.U., Gruber W.C. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. - DOI - PMC - PubMed
-
- Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., Bibi S., Briner C., Cicconi P., Collins A.M., Colin-Jones R., Cutland C.L., Darton T.C., Dheda K., Duncan C.J.A., Emary K.R.W., Ewer K.J., Fairlie L., Faust S.N., Feng S., Ferreira D.M., Finn A., Goodman A.L., Green C.M., Green C.A., Heath P.T., Hill C., Hill H., Hirsch I., Hodgson S.H.C., Izu A., Jackson S., Jenkin D., Joe C.C.D., Kerridge S., Koen A., Kwatra G., Lazarus R., Lawrie A.M., Lelliott A., Libri V., Lillie P.J., Mallory R., Mendes A.V.A., Milan E.P., Minassian A.M., McGregor A., Morrison H., Mujadidi Y.F., Nana A., O’Reilly P.J., Padayachee S.D., Pittella A., Plested E., Pollock K.M., Ramasamy M.N., Rhead S., Schwarzbold A.V., Singh N., Smith A., Song R., Snape M.D., Sprinz E., Sutherland R.K., Tarrant R., Thomson E.C., Török M.E., Toshner M., Turner D.P.J., Villafana T.L., Watson M.E.E., Williams C.J., Douglas A.D., Hill A.V.S., Lambe T., Gilbert S.C., Pollard A.J. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

