SABR for High-Risk Prostate Cancer: A Prospective Multilevel MRI-Based Dose Escalation Trial
- PMID: 34774676
- PMCID: PMC9091061
- DOI: 10.1016/j.ijrobp.2021.10.137
SABR for High-Risk Prostate Cancer: A Prospective Multilevel MRI-Based Dose Escalation Trial
Abstract
Purpose: Radiation dose intensification improves outcome in men with high-risk prostate cancer (HR-PCa). A prospective trial was conducted to determine safety, feasibility, and maximal tolerated dose of multilevel magnetic resonance imaging (MRI)-based 5-fraction SABR in patients with HR-PCa.
Methods and materials: This phase I clinical trial enrolled patients with HR-PCa with grade group ≥4, prostate-specific antigen (PSA) ≥20 ng/mL, or radiographic ≥T3, and well-defined prostatic lesions on multiparametric MRI (mpMRI) into 4 dose-escalation cohorts. The initial cohort received 47.5 Gy to the prostate, 50 Gy to mpMRI-defined intraprostatic lesion(s), and 22.5 Gy to pelvic lymph nodes in 5 fractions. Radiation doses were escalated for pelvic nodes to 25 Gy and mpMRI lesion(s) to 52.5 Gy and then 55 Gy. Escalation was performed sequentially according to rule-based trial design with 7 to 15 patients per cohort and a 90-day observation period. All men received peri-rectal hydrogel spacer, intraprostatic fiducial placement, and 2 years of androgen deprivation. The primary endpoint was maximal tolerated dose according to a 90-day acute dose-limiting toxicity (DLT) rate <33%. DLT was defined as National Cancer Institute Common Toxicity Criteria for Adverse Events ≥grade 3 treatment-related toxicity. Secondary outcomes included acute and delayed gastrointestinal (GI)/genitourinary (GU) toxicity graded with Common Toxicity Criteria for Adverse Events.
Results: Fifty-five of the 62 enrolled patients were included in the analysis. Dose was escalated through all 4 cohorts without observing any DLTs. Median overall follow-up was 18 months, with a median follow-up of 42, 24, 12, and 7.5 months for cohorts 1 to 4 respectively. Acute and late grade 2 GU toxicities were 25% and 20%, while GI were 13% and 7%, respectively. Late grade 3 GU and GI toxicities were 2% and 0%, respectively.
Conclusions: SABR dose for HR-PCa was safely escalated with multilevel dose painting of 47.5 Gy to prostate, 55 Gy to mpMRI-defined intraprostatic lesions, and 25 Gy to pelvic nodal region in 5 fractions. Longer and ongoing follow-up will be required to assess late toxicity.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest Statement for All Authors
The remaining authors do not have any conflicts of interest to report.
Figures
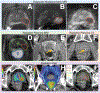

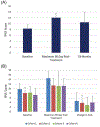
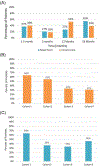
Comment in
-
Are We Ready for Focal Dose Radio-Ablation in the Treatment of Localized Prostate Cancer?Int J Radiat Oncol Biol Phys. 2022 Jun 1;113(2):302-304. doi: 10.1016/j.ijrobp.2022.02.032. Int J Radiat Oncol Biol Phys. 2022. PMID: 35569475 No abstract available.
Similar articles
-
Phase I trial of pelvic nodal dose escalation with hypofractionated IMRT for high-risk prostate cancer.Int J Radiat Oncol Biol Phys. 2012 Jan 1;82(1):184-90. doi: 10.1016/j.ijrobp.2010.09.018. Epub 2010 Dec 14. Int J Radiat Oncol Biol Phys. 2012. PMID: 21163590 Free PMC article. Clinical Trial.
-
Hypofractionated, Dose Escalation Radiation Therapy for High-Risk Prostate Cancer: The Safety Analysis of the Prostate Cancer Study-5, a Groupe de Radio-Oncologie Génito-Urinaire de Quebec Led Phase 3 Trial.Int J Radiat Oncol Biol Phys. 2024 Jan 1;118(1):52-62. doi: 10.1016/j.ijrobp.2023.05.014. Epub 2023 May 22. Int J Radiat Oncol Biol Phys. 2024. PMID: 37224928 Clinical Trial.
-
50-Gy Stereotactic Body Radiation Therapy to the Dominant Intraprostatic Nodule: Results From a Phase 1a/b Trial.Int J Radiat Oncol Biol Phys. 2019 Feb 1;103(2):320-334. doi: 10.1016/j.ijrobp.2018.09.023. Epub 2018 Sep 27. Int J Radiat Oncol Biol Phys. 2019. PMID: 30267761 Clinical Trial.
-
Safety of Ultrahypofractionated Pelvic Nodal Irradiation in the Definitive Management of Prostate Cancer: Systematic Review and Meta-analysis.Int J Radiat Oncol Biol Phys. 2024 Mar 15;118(4):998-1010. doi: 10.1016/j.ijrobp.2023.09.053. Epub 2023 Oct 19. Int J Radiat Oncol Biol Phys. 2024. PMID: 37863241
-
Magnetic resonance imaging (MRI)-based radiomics for prostate cancer radiotherapy.Transl Androl Urol. 2018 Jun;7(3):445-458. doi: 10.21037/tau.2018.06.05. Transl Androl Urol. 2018. PMID: 30050803 Free PMC article. Review.
Cited by
-
Focal Boost in Prostate Cancer Radiotherapy: A Review of Planning Studies and Clinical Trials.Cancers (Basel). 2023 Oct 8;15(19):4888. doi: 10.3390/cancers15194888. Cancers (Basel). 2023. PMID: 37835581 Free PMC article. Review.
-
Stereotactic Radiation Therapy versus Brachytherapy: Relative Strengths of Two Highly Efficient Options for the Treatment of Localized Prostate Cancer.Cancers (Basel). 2022 Apr 29;14(9):2226. doi: 10.3390/cancers14092226. Cancers (Basel). 2022. PMID: 35565355 Free PMC article. Review.
-
Stereotactic Body Radiotherapy: Hitting Harder, Faster, and Smarter in High-Risk Prostate Cancer.Front Oncol. 2022 Jul 7;12:889132. doi: 10.3389/fonc.2022.889132. eCollection 2022. Front Oncol. 2022. PMID: 35875062 Free PMC article. Review.
-
Toxicity profile and clinical outcomes of stereotactic body radiotherapy with a focal boost without fiducials or perirectal hydrogel spacer for localized prostate cancer.Strahlenther Onkol. 2025 Aug;201(8):779-787. doi: 10.1007/s00066-024-02333-4. Epub 2024 Dec 10. Strahlenther Onkol. 2025. PMID: 39656294
-
Functional imaging guided stereotactic ablative body radiotherapy (SABR) with focal dose escalation and bladder trigone sparing for intermediate and high-risk prostate cancer: study protocol for phase II safo trial.Radiat Oncol. 2024 May 3;19(1):54. doi: 10.1186/s13014-024-02440-7. Radiat Oncol. 2024. PMID: 38702761 Free PMC article.
References
-
- Zaorsky NG, Trabulsi EJ, Lin J, et al. Multimodality therapy for patients with high-risk prostate cancer: Current status and future directions. Semin Oncol 2013;40:308–321. - PubMed
-
- Zaorsky NG, Palmer JD, Hurwitz MD, et al. What is the ideal radio-therapy dose to treat prostate cancer? A meta-analysis of biologically equivalent dose escalation. Radiother Oncol 2015;115:295–300. - PubMed
-
- Kerkmeijer LGW, Groen VH, Pos FJ, et al. Focal boost to the intrapro-static tumor in external beam radiotherapy for patients with localized prostate cancer: Results from the flame randomized phase III trial. J Clin Oncol 2021. JCO2002873. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

