Assessment of ACE1 variants and ACE1/ACE2 expression in COVID-19 patients
- PMID: 34774774
- PMCID: PMC8579741
- DOI: 10.1016/j.vph.2021.106934
Assessment of ACE1 variants and ACE1/ACE2 expression in COVID-19 patients
Abstract
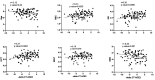
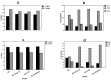
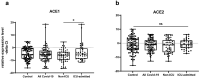
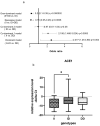
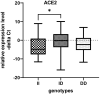
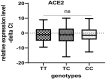
Contribution of the renin-angiotensinogen system in the risk of COVID-19 and related complications have been assessed by several groups. However, the results are not consistent. We examined levels of ACE1 and ACE2 in the circulation of two groups of COVID-19 patients (ICU-admitted and general ward-admitted patients) compared with healthy controls. We also genotyped two polymorphisms in ACE1 gene (the ACE1-I/D polymorphism rs1799752 and rs4359) to appraise their association with expression levels of ACE1 and ACE2. Expression level of ACE1 was significantly higher in ICU patients compared with non-ICU patients (P value = 0.02). However, its expression was not significantly different between total COVID-19 patients and total controls (P value = 0.34). ACE2 expression was not different ether between two groups of COVID-19 patients (P value = 0.12) or between total COVID-19 patients and total controls (P value = 0.79). While distribution of rs1799752 and rs4359 alleles was similar between study groups, genotype frequencies of rs1799752 were differently distributed among total COVID-19 patients and controls (P value = 0.00001). Moreover, genotypes of the other polymorphism tended to be distinctively distributed among these two groups (P value = 0.06). In the total population of patients and controls, different ACE1 mRNA levels were observed among carriers of different rs1799752 genotypes; of note, ID genotype carriers showed a higher expression of ACE1 compared with II genotype carriers (P = 0.01). ACE1 polymorphisms might affect risk of COVID-19 and expression of ACE transcripts.
Keywords: ACE1; ACE2; COVID-19; Expression.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare they have no conflict of interest.
Figures


References
-
- Tatullo M., Codispoti B., Pacifici A., Palmieri F., Marrelli M., Pacifici L., et al. Potential use of human periapical cyst-mesenchymal stem cells (hPCy-MSCs) as a novel stem cell source for regenerative medicine applications. Front. Cell Develop. Biol. 2017;5:103. PubMed PMID: 29259970. eng. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

