Forward and reverse translational approaches to predict efficacy of neutralizing respiratory syncytial virus (RSV) antibody prophylaxis
- PMID: 34775220
- PMCID: PMC8603022
- DOI: 10.1016/j.ebiom.2021.103651
Forward and reverse translational approaches to predict efficacy of neutralizing respiratory syncytial virus (RSV) antibody prophylaxis
Abstract
Background: Neutralizing mAbs can prevent communicable viral diseases. MK-1654 is a respiratory syncytial virus (RSV) F glycoprotein neutralizing monoclonal antibody (mAb) under development to prevent RSV infection in infants. Development and validation of methods to predict efficacious doses of neutralizing antibodies across patient populations exposed to a time-varying force of infection (i.e., seasonal variation) are necessary.
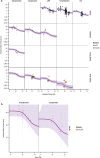
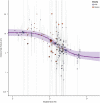
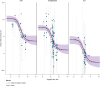
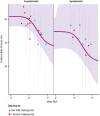
Methods: Five decades of clinical trial literature were leveraged to build a model-based meta-analysis (MBMA) describing the relationship between RSV serum neutralizing activity (SNA) and clinical endpoints. The MBMA was validated by backward translation to animal challenge experiments and forward translation to predict results of a recent RSV mAb trial. MBMA predictions were evaluated against a human trial of 70 participants who received either placebo or one of four dose-levels of MK-1654 and were challenged with RSV [NCT04086472]. The MBMA was used to perform clinical trial simulations and predict efficacy of MK-1654 in the infant target population.
Findings: The MBMA established a quantitative relationship between RSV SNA and clinical endpoints. This relationship was quantitatively consistent with animal model challenge experiments and results of a recently published clinical trial. Additionally, SNA elicited by increasing doses of MK-1654 in humans reduced RSV symptomatic infection rates with a quantitative relationship that approximated the MBMA. The MBMA indicated a high probability that a single dose of ≥ 75 mg of MK-1654 will result in prophylactic efficacy (> 75% for 5 months) in infants.
Interpretation: An MBMA approach can predict efficacy of neutralizing antibodies against RSV and potentially other respiratory pathogens.
Keywords: Human Challenge Study; Modelling and Simulation; Monoclonal Antibody; RSV, Meta-analysis; Respiratory Syncytial Virus.
Copyright © 2021 Merck Sharp & Dohme Corp., The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest BM, RR, LC, JC, WL, YZ, QH, WG, DS, BR, AE, SS, EL, AA and KV are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA (or were at the time of the study), and may hold stock in Merck & Co., Inc., Kenilworth, NJ, USA. JRS is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and may hold stock in Merck & Co., Inc., Kenilworth, NJ, USA and reports other investments that are less than 1% ownership for any company. JL, NP, LQ, HW, ES, BG, and FB are employed by Certara, Princeton, NJ, USA (or were employed at the time of the study) and may hold shares in Certara, Princeton, NJ, USA. Certara received funding from Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, for modelling work. ASYC is employed by Certara, Princeton, NJ, USA and holds stock in Certara, Princeton, NJ, USA and AstraZeneca, Cambridge, UK and is a chair of IQ consortium TALG and CLPG Pediatric PBPK group. JM, MK, AP, and JFK : nothing to disclose.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

