Italian experience with rVIII-single chain: a survey of patients with haemophilia A and their physicians
- PMID: 34775566
- PMCID: PMC9148280
- DOI: 10.1007/s11239-021-02599-w
Italian experience with rVIII-single chain: a survey of patients with haemophilia A and their physicians
Abstract
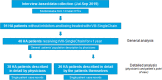
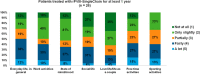
rVIII-SingleChain is indicated for treatment and prophylaxis of bleeding in patients with haemophilia A (HA). The safety and efficacy of rVIII-SingleChain have previously been shown in the AFFINITY clinical trial programme. This survey evaluated clinical experience following a switch to rVIII-SingleChain from the perspective of both physicians and patients. A web-based survey (July-September 2019) involving 14 Haemophilia Treatment Centres (HTCs) collected data about HA patients who were under treatment with rVIII-SingleChain for ≥ 12 months, as reported by their physicians. In addition, about half of these patients were separately interviewed. Out of 91 patients receiving rVIII-SingleChain in the 14 participating HTCs, 48 had been treated for ≥ 12 months; among those 48, 38% were ≤ 18 years, 37% 19-40 years and 25 % ≥ 41 years; 73% of them had severe HA and 85% were being treated with prophylactic therapy. Twenty-six patients accepted to be separately interviewed: mean age was 30 years; 62% had severe HA and 85% were receiving prophylaxis. Focusing on those patients who were already in prophylaxis with prior FVIII (all but one with recombinant factors), infusion frequency was significantly reduced from 3-2 per week following the switch to rVIII-SingleChain (mean, 2.74 vs. 2.44, respectively; p=0.013), as reported by physicians; the rate of patients needing 3 infusions per week dropped from 74% with previous products to 44% with rFVIII-SingleChain. The annual mean factor consumption was 4740 IU/Kg (median, 4500 IU/Kg; min, 2.215 IU/Kg; max, 7.200 IU/Kg) with prior product and 4320 IU/Kg (median, 4320 IU/Kg; min, 2.215 IU/Kg; max, 6.646 IU/Kg) with rVIII-SingleChain. Both physicians and patients reported a significant reduction in annual total bleeding rates with rVIII-SingleChain compared with prior product (mean 2.15-0.96 and 2.46-0.71 events/year, p = 0.031 and p = 0.018, respectively). Mean satisfaction ratings (from 1; dissatisfied, to 5; very satisfied) for rVIII-SingleChain were quite high for both physicians (4.14, 86% satisfied/very satisfied) and patients (4.18, 86% satisfied/very satisfied). This survey suggested that switching to rVIII-SingleChain allowed patients to reduce their injection frequency without increasing factor consumption or compromising clinical results. Both physicians and patients reported a positive experience with rVIII-SingleChain after 1 year of treatment.
Keywords: Haemophilia A; Injection frequency; rVIII-SingleChain.
© 2021. The Author(s).
Conflict of interest statement
AB received honoraria for speaker’s bureau and/or participation in Advisory Boards from Bayer, CSL Behring, Kedrion, Novo Nordisk, Roche, Sobi, Takeda. GC received honoraria for speaker’s bureau and/or participation in Advisory Boards for Alexion. Bayer, Biomarin, CSL Behring, Grifols, Kedrion, LFB, NovoNordisk, Roche, Sanofi, Sobi, Takeda, Uniqure, Werfen. AR acted as paid consultant/member of Advisory Board/speaker for Bayer, CSL Behring, Kedrion, NovoNordisk, Roche, Sobi, Takeda. CS received honoraria for speaker’s bureau and/or participation in Advisory Boards from Bayer, Takeda, CSL Behring, Sobi, Roche, NovoNordisk.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical