Whole blood-based measurement of SARS-CoV-2-specific T cells reveals asymptomatic infection and vaccine immunogenicity in healthy subjects and patients with solid-organ cancers
- PMID: 34775604
- PMCID: PMC8653009
- DOI: 10.1111/imm.13433
Whole blood-based measurement of SARS-CoV-2-specific T cells reveals asymptomatic infection and vaccine immunogenicity in healthy subjects and patients with solid-organ cancers
Abstract
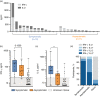
Accurate assessment of SARS-CoV-2 immunity is critical in evaluating vaccine efficacy and devising public health policies. Whilst the exact nature of effective immunity remains incompletely defined, SARS-CoV-2-specific T-cell responses are a critical feature that will likely form a key correlate of protection against COVID-19. Here, we developed and optimized a high-throughput whole blood-based assay to determine the T-cell response associated with prior SARS-CoV-2 infection and/or vaccination amongst 231 healthy donors and 68 cancer patients. Following overnight in vitro stimulation with SARS-CoV-2-specific peptides, blood plasma samples were analysed for TH 1-type cytokines. Highly significant differential IFN-γ+ /IL-2+ SARS-CoV-2-specific T-cell responses were seen amongst previously infected COVID-19-positive healthy donors in comparison with unknown / naïve individuals (p < 0·0001). IFN-γ production was more effective at identifying asymptomatic donors, demonstrating higher sensitivity (96·0% vs. 83·3%) but lower specificity (84·4% vs. 92·5%) than measurement of IL-2. A single COVID-19 vaccine dose induced IFN-γ and/or IL-2 SARS-CoV-2-specific T-cell responses in 116 of 128 (90·6%) healthy donors, reducing significantly to 27 of 56 (48·2%) when measured in cancer patients (p < 0·0001). A second dose was sufficient to boost T-cell responses in the majority (90·6%) of cancer patients, albeit IFN-γ+ responses were still significantly lower overall than those induced in healthy donors (p = 0·034). Three-month post-vaccination T-cell responses also declined at a faster rate in cancer patients. Overall, this cost-effective standardizable test ensures accurate and comparable assessments of SARS-CoV-2-specific T-cell responses amenable to widespread population immunity testing, and identifies individuals at greater need of booster vaccinations.
Keywords: COVID-19; SARS-CoV-2; T cells; antibodies; vaccine.
© 2021 The Authors. Immunology published by John Wiley & Sons Ltd.
Conflict of interest statement
MJS is a founder of and holds equity in ImmunoServ Ltd. AnG is a founder of ImmunoServ Ltd. All other authors declare no conflicts of interest.
Figures


References
-
- Le Bert N, Tan AT, Kunasegaran K, Tham CYL, Hafezi M, Chia A, et al. SARS‐CoV‐2‐specific T cell immunity in cases of COVID‐19 and SARS, and uninfected controls. Nature. 2020;584:457–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

